Abstract
Background
The mutations in the presenilin 1 gene (PSEN1) are the main cause of familial Alzheimer's disease. PSEN1 mutations affect amyloid-beta peptide production, which accumulates in the brain as senile plaque and cotton wool plaques (CWPs) and relates to other neurodegenerative disorders. Here we report the second case of the PSEN1 G266S mutation, which showed distinctive neuropathological features, including abundant CWPs. Lewy body pathology, and altered amyloid-beta production.
Method
Using the proband’s samples, we performed genetic analysis of the PSEN1, APP, MAPT, and APOE genes, histopathological and immunohistochemical analysis of the brain tissue, and biochemical analysis of Aβ production in COS cells transfected with wild-type or mutant PSEN1.
Results
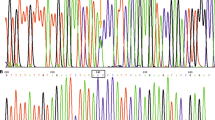
The patient presented with memory loss, abnormal behavior, and visual hallucinations. Brain scans showed reduced blood flow, mild atrophy, and white matter lesions. Genetic analysis revealed a heterozygous mutation at codon 266 (G266S) of PSEN1 and polymorphism of MAPT (Q230R). The brain had many CWPs, severe cerebral amyloid angiopathy (CAA), senile plaque, Lewy bodies, and neurites. Electron microscopy displayed myelinated fiber degeneration, mitochondrial damage, and amyloid fibrils in the white matter. The production level of Aβ42 in PSEN1 G266S-transfected cells significantly increased.
Conclusion
Our findings suggest that the PSEN1 G266S mutation may cause a heterogeneous clinical and pathological phenotype, influenced by other genetic or environmental factors.





Similar content being viewed by others
References
Breijyeh Z, Karaman R (2020) Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules 25:5789. https://doi.org/10.3390/molecules25245789
Van Giau V, Pyun JM, Suh J, Bagyinszky E, An SSA, Kim SY (2019) A pathogenic PSEN1 Trp165Cys mutation associated with early-onset Alzheimer’s disease. BMC Neurol 19:188. https://doi.org/10.1186/s12883-019-1419-y
Crook R, Verkkoniemi A, Perez-Tur J et al (1998) A variant of Alzheimer’s disease with spastic paraparesis and unusual plaques due to deletion of exon 9 of presenilin 1. Nat Med 4:452–455. https://doi.org/10.1038/nm0498-452
Ryan NS, Nicholas JM, Weston PSJ et al (2016) Clinical phenotype and genetic associations in autosomal dominant familial Alzheimer’s disease: a case series. Lancet Neurol 15:1326–1335. https://doi.org/10.1016/S1474-4422(16)30193-4
Matsubara-Tsutsui M, Yasuda M, Yamagata H et al (2002) Molecular evidence of presenilin 1 mutation in familial early onset dementia. Am J Med Genet 114:292–298. https://doi.org/10.1002/ajmg.10250
Hutton M, Busfield F, Wragg M et al (1996) Complete analysis of the presenilin 1 gene in early onset Alzheimer’s disease. NeuroReport 7:801–805. https://doi.org/10.1097/00001756-199602290-00029
Yoshiiwa A, Kamino K, Yamamoto H et al (1997) alpha 1-Antichymotrypsin as a risk modifier for late-onset Alzheimer’s disease in Japanese apolipoprotein E epsilon 4 allele carriers. Ann Neurol 42:115–117. https://doi.org/10.1002/ana.410420118
Mullan M, Crawford F, Axelman K et al (1992) A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet 1:1345–1347. https://doi.org/10.1038/ng0892-345
Lewis PA, Perez-Tur J, Golde TE, Hardy J (2000) The presenilin 1 C92S mutation increases abeta 42 production. Biochem Biophys Res Commun 277:261–263. https://doi.org/10.1006/bbrc.2000.3646
Kelleher RJ 3rd, Shen J (2017) Presenilin-1 mutations and Alzheimer’s disease. Proc Natl Acad Sci U S A 114:629–631. https://doi.org/10.1073/pnas.1619574114
Yang Y, Bagyinszky E, An SSA (2023) Presenilin-1 (PSEN1) Mutations: Clinical Phenotypes beyond Alzheimer’s Disease. Int J Mol Sci 24:8417. https://doi.org/10.3390/ijms24098417
Bagaria J, Bagyinszky E, An SSA (2022) Genetics, functions, and clinical impact of presenilin-1 (PSEN1) gene. Int J Mol Sci 23:10970. https://doi.org/10.3390/ijms231810970
Farrer LA, Cupples LA, Haines JL et al (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer disease meta analysis consortium. JAMA 278:1349–1356
Bu G (2009) Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 10:333–344. https://doi.org/10.1038/nrn2620
Costa-Laparra I, Juárez-Escoto E, Vicario C, Moratalla R, García-Sanz P (2023) APOE ε4 allele, along with G206D-PSEN1 mutation, alters mitochondrial networks and their degradation in Alzheimer’s disease. Front Aging Neurosci 15:1087072. https://doi.org/10.3389/fnagi.2023.1087072
Willumsen N, Poole T, Nicholas JM, Fox NC, Ryan NS, Lashley T (2022) Variability in the type and layer distribution of cortical Aβ pathology in familial Alzheimer’s disease. Brain Pathol 32:e13009. https://doi.org/10.1111/bpa.13009
Andreadis A, Brown WM, Kosik KS (1992) Structure and novel exons of the human tau gene. Biochemistry 31:10626–10633. https://doi.org/10.1021/bi00158a027
Goedert M, Spillantini MG, Crowther RA (1983) Cloning of a big tau microtubule-associated protein characteristic of the peripheral nervous system. Proc Natl Acad Sci U S A 89:1983–1987. https://doi.org/10.1073/pnas.89.5.1983
Jin SC, Pastor P, Cooper B, Cervantes S et al (2012) Pooled-DNA sequencing identifies novel causative variants in PSEN1, GRN and MAPT in a clinical early-onset and familial Alzheimer’s disease Ibero-American cohort. Alzheimers Res Ther 4:34. https://doi.org/10.1186/alzrt137
Acknowledgments
This work was supported by a grant from the Japanese Millennium Project. We thank Norihiro Ogawa for technical assistance in the pathological study. We are most grateful to all participants in the study.
Author information
Authors and Affiliations
Contributions
HDY: study design, genetic analysis. HA, AW, IW, NK, TeM: data collection. TF: drafting and revising manuscript. KK, TaM, TY, AH, MM, MY, YH: pathological studies. NS: functional assay. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures used in this research were approved by the Ethics Committee of Ehime University School of Medicine and were conducted according to the principles of the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individuals included in this study.
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamagata, H.D., Akatsu, H., Fukuoka, T. et al. Novel insights into presenilin 1 mutation associated with a distinctive dementia phenotype and cotton wool plaques. Neurol Sci (2024). https://doi.org/10.1007/s10072-024-07537-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10072-024-07537-1