Abstract
Objective
The relationship between the cell body layer and the dendritic network layer of the retina and cognitive performance (CP) in MS patients has not been examined separately. The objective of this study is to predict cognitive impairment (CI) in RRMS patients and to examine the relationship between CP and ganglion cell layer (GCL), inner plexiform layer (IPL), and GCL divided by IPL (GCL/IPL).
Methods
Ophthalmological evaluation, retinal segmentation, and Symbol Digit Modalities Test (SDMT) were performed on 102 RRMS patients and 54 healthy subjects. The relationships of GCL, IPL, and GCL/IPL with CP in eyes without a history of optic neuritis were investigated using Spearman’s correlation. Models were created by accepting 1 standard deviation less of the SDMT mean of the control group as the limit for CI. The cutoff value of the GCL/IPL variable that could predict CI was calculated by ROC analysis, and the ability to accurately predict CI was tested with binary logistic regression.
Results
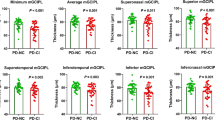
No correlation was found between OCT parameters and CP in healthy subjects. Correlation was found between GCL thickness and GCL/IPL variable and CP in RRMS patients (r=0.235, r=0.667 respectively). A GCL/IPL value of 1.255 was able to identify CI with 81.8% sensitivity and 75.9% specificity (AUC=0.844, LR=3.38) and predicted CI with 74.5% accuracy (Nagelkerke R2=0.439).
Conclusion
In RRMS patients, the IPL thickness is unrelated to CP. Therewithal, the GCL/IPL-CP relationship is stronger than the GCL-CP relationship and GCL/IPL can predict CI.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AUC:
-
Area under curve
- CI:
-
Cognitive impairment
- CP:
-
Cognitive performance
- CNS:
-
Central nervous system
- EDSS:
-
Expanded Disability Status Scale
- ETDRS:
-
Early Treatment Diabetic Retinopathy Study
- GCL:
-
Ganglion cell layer
- IPL:
-
Inner plexiform layer
- GCL/IPL:
-
GCL divided by IPL
- INL:
-
Inner nuclear layer
- LR:
-
Likelihood ratio
- MCI:
-
Mild cognitive impairment
- OCT:
-
Optical coherence tomography
- ON:
-
Optic neuritis
- RNFL:
-
Retinal nerve fiber layer
- ROC:
-
Receiver operating characteristics
- RRMS:
-
Relapsing remitting multiple sclerosis
- SD:
-
Standard deviation
- SDMT:
-
Symbol Digit Modalities Test
References
Feinstein A (2021) Wordsworth, Bellow, and understanding multiple sclerosis. Lancet Neurol 20:177–178. https://doi.org/10.1016/S1474-4422(19)30222-4
Van Schependom J, D’hooghe MB, Cleynhens K et al (2014) The Symbol Digit Modalities Test as sentinel test for cognitive impairment in multiple sclerosis. Eur J Neurol 21. https://doi.org/10.1111/ene.12463
Benedict RHB, Deluca J, Phillips G et al (2017) Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler 23:721–733
Strober L, DeLuca J, Benedict RHB et al (2019) Symbol Digit Modalities Test: a valid clinical trial endpoint for measuring cognition in multiple sclerosis. Mult Scler J 25:1781–1790. https://doi.org/10.1177/1352458518808204
Amato MP, Portaccio E, Goretti B et al (2010) Relevance of cognitive deterioration in early relapsing-remitting MS: a 3-year follow-up study. Mult Scler 16:1474–1482. https://doi.org/10.1177/1352458510380089
Esmael A, Elsherif M, Abdelsalam M et al (2020) Retinal thickness as a potential biomarker of neurodegeneration and a predictor of early cognitive impairment in patients with multiple sclerosis. Neurol Res 42:564–574. https://doi.org/10.1080/01616412.2020.1761174
Jakimovski D, Benedict RHB, Weinstock-Guttman B et al (2021) Visual deficits and cognitive assessment of multiple sclerosis: confounder, correlate, or both? J Neurol. https://doi.org/10.1007/s00415-021-10437-5
Bsteh G, Hegen H, Teuchner B et al (2019) Peripapillary retinal nerve fibre layer as measured by optical coherence tomography is a prognostic biomarker not only for physical but also for cognitive disability progression in multiple sclerosis. Mult Scler J 25:196–203. https://doi.org/10.1177/1352458517740216
Coric D, Balk LJ, Verrijp M et al (2018) Cognitive impairment in patients with multiple sclerosis is associated with atrophy of the inner retinal layers. Mult Scler 24:158–166. https://doi.org/10.1177/1352458517694090
Kulkarni VA, Firestein BL (2012) The dendritic tree and brain disorders. Mol Cell Neurosci 50:10–20. https://doi.org/10.1016/J.MCN.2012.03.005
Reza-Zaldivar EE, Hernández-Sápiens MA, Minjarez B et al (2020) Dendritic spine and synaptic plasticity in Alzheimer’s disease: a focus on MicroRNA. Front Cell Dev Biol 8:1–9. https://doi.org/10.3389/fcell.2020.00255
Hammond JW, Bellizzi MJ, Ware C et al (2020) Complement-dependent synapse loss and microgliosis in a mouse model of multiple sclerosis. Brain Behav Immun 87:739–750. https://doi.org/10.1016/j.bbi.2020.03.004
Jafari M, Schumacher AM, Snaidero N et al (2021) Phagocyte-mediated synapse removal in cortical neuroinflammation is promoted by local calcium accumulation. Nat Neurosci 24:355–367. https://doi.org/10.1038/s41593-020-00780-7
Tewarie P, Balk L, Costello F et al (2012) The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One 7. https://doi.org/10.1371/JOURNAL.PONE.0034823
Early Treatment Diabetic Retinopathy Study research group (1985) Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 103:1796–1806
Nolan-Kenney RC, Liu M, Akhand O et al (2019) Optimal intereye difference thresholds by optical coherence tomography in multiple sclerosis: an international study. Ann Neurol 85:618–629. https://doi.org/10.1002/ana.25462
Gabilondo I, Martínez-Lapiscina EH, Martínez-Heras E et al (2014) Trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann Neurol 75:98–107. https://doi.org/10.1002/ana.24030
Xu SC, Kardon RH, Leavitt JA et al (2019) Optical coherence tomography is highly sensitive in detecting prior optic neuritis. Neurology 92:e527–e535. https://doi.org/10.1212/WNL.0000000000006873
Interocular difference in retinal nerve fiber layer thickness predicts optic neuritis in pediatric-onset multiple sclerosis - PubMed. https://pubmed.ncbi.nlm.nih.gov/33105412/. Accessed 14 Apr 2021
Pisa M, Croese T, Dalla Costa G et al (2021) Subclinical anterior optic pathway involvement in early multiple sclerosis and clinically isolated syndromes. Brain 144:848–862. https://doi.org/10.1093/brain/awaa458
Coombs J, van der List D, Wang G-Y, Chalupa LM (2006) Morphological properties of mouse retinal ganglion cells. Neuroscience 140:123–136. https://doi.org/10.1016/J.NEUROSCIENCE.2006.02.079
Kong J-H, Fish DR, Rockhill RL, Masland RH (2005) Diversity of ganglion cells in the mouse retina: unsupervised morphological classification and its limits. J Comp Neurol 489:293–310. https://doi.org/10.1002/CNE.20631
Sun W, Li N, He S (2002) Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol 451:115–126. https://doi.org/10.1002/CNE.10323
Rockhill RL, Daly FJ, MacNeil MA et al (2002) The diversity of ganglion cells in a mammalian retina. J Neurosci 22:3831–3843. https://doi.org/10.1523/JNEUROSCI.22-09-03831.2002
Citron MC, Emerson RC, Levick WR (1988) Nonlinear measurement and classification of receptive fields in cat retinal ganglion cells. Ann Biomed Eng 16:65–77. https://doi.org/10.1007/BF02367381
Santina LD, Inman DM, Lupien CB et al (2013) Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. J Neurosci 33:17444–17457. https://doi.org/10.1523/JNEUROSCI.5461-12.2013
Subramaniam S (2019) Selective neuronal death in neurodegenerative diseases: the ongoing mystery. Yale J Biol Med 92(4):695–705
Schirmer L, Velmeshev D, Holmqvist S et al (2019) Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 573:75–82. https://doi.org/10.1038/S41586-019-1404-Z
Mayer C, Bruehl C, Salt EL et al (2018) Selective vulnerability of αOFF retinal ganglion cells during onset of autoimmune optic neuritis. Neuroscience 393:258–272. https://doi.org/10.1016/J.NEUROSCIENCE.2018.07.040
Azevedo CJ, Overton E, Khadka S et al (2015) Early CNS neurodegeneration in radiologically isolated syndrome. Neurol(R) Neuroimmunology Neuroinflammation 2:e102. https://doi.org/10.1212/NXI.0000000000000102
Cilingir V, Batur M (2020) Axonal degeneration independent of inflammatory activity: is it more intense in the early stages of relapsing-remitting multiple sclerosis disease? Eur Neurol 83(5):508–516. https://doi.org/10.1159/000510116
Cilingir V, Akdeniz H (2021) The course of cervical spinal cord atrophy rate and its relationship with NEDA in relapsing remitting multiple sclerosis. Acta Neurol Belg. https://doi.org/10.1007/s13760-021-01595-4
Bsteh G, Berek K, Hegen H et al (2020) Macular ganglion cell–inner plexiform layer thinning as a biomarker of disability progression in relapsing multiple sclerosis. Mult Scler J:1–11. https://doi.org/10.1177/1352458520935724
Noah AM, Almghairbi D, Moppett IK (2020) Optical coherence tomography in mild cognitive impairment – systematic review and meta-analysis. Clin Neurol Neurosurg 196:106036. https://doi.org/10.1016/j.clineuro.2020.106036
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study has been approved by the clinical research ethical committee of faculty of medicine-Van Yuzuncu Yil university.
Informed consent
All participants have provided an informed written consent before participation in this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cilingir, ., Seven, E. Retinal clues for selective neuronal loss in multiple sclerosis. Neurol Sci 45, 1163–1171 (2024). https://doi.org/10.1007/s10072-023-07110-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07110-2