Abstract
Objectives
We aimed to evaluate the available evidence on the efficacy and safety outcomes of intravenous tenecteplase (TNK) compared with intravenous alteplase(ALT) for patients with acute ischemic stroke (AIS) in randomized controlled trials (RCTs).
Methods
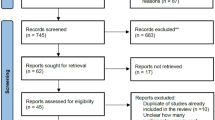
The MEDLINE/PubMed, Embase, Springer, Web of Science, Cochrane Collaboration database, China National Knowledge Infrastructure (CNKI) database, and Wanfang database were comprehensively searched for RCTs regarding the effects of TNK versus ALT among AIS patients in these English and Chinese electronic databases from inception dates to August 1, 2022. This meta-analysis followed PRISMA guidelines. Two reviewers independently retrieved RCTs and extracted relevant information. The methodological quality of the included trials was estimated using the Cochrane risk of bias tool. The pooled analyses were performed using RevMan 5.3 software. The primary outcome was functional outcome on the modified Rankin Scale (mRS) (range 0 to 5) and mortality at 90 days. The secondary outcomes included successful recanalization, early neurologic improvement < 48 h, any intracranial hemorrhage (ICH), and symptomatic ICH. The follow-up time of all studies was at least 3 months.
Results
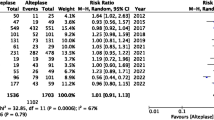
A total of nine RCTs involving 1958 patients in TNK group and 1731 patients in ALT group were finally included. For the efficacy outcomes, there were no significant differences between the two groups in terms of mRS score 0 ~ 2 (RR 1.00; 95% CI 0.88–1.13; P = 0.96), mRS score 0 ~ 1 (RR 1.03; 95% CI 0.96–1.10; P = 0.36), successful recanalization (RR 1.25; 95% CI 0.88–1.76; P = 0.21), and early neurologic improvement < 48 h (RR 1.08; 95% CI 0.92–1.26; P = 0.37). Similar results were seen for the safety outcomes, which have no statistical differences in terms of any ICH (RR 1.01; 95% CI 0.72–1.41; P = 0.96), symptomatic ICH (RR 1.19; 95% CI 0.81–1.76; P = 0.37), and mortality at 90 days (RR 0.99; 95% CI 0.83–1.19; P = 0.94).
Conclusion
Overall, the efficacy and safety outcomes of intravenous thrombolysis with TNK versus ALT for AIS were not statistically different. However, TNK at a dose of 0.25 mg/kg may be a reasonable alternative to ALT for thrombolysis.











Similar content being viewed by others
References
Powers WJ (2020) Acute ischemic stroke. N Engl J Med 383(3):252–260
Powers WJ, Rabinstein AA, Ackerson T et al (2018) 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49(3):e46–e110
Bhatia R, Hill MD, Shobha N et al (2010) Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 41(10):2254–2258
Tanswell P, Modi N, Combs D et al (2002) Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet 41:1229–1245
Cannon CP, Gibson CM, McCabe CH et al (1998) TNK-tissue plasminogen activator compared with front-loaded alteplase in acute myocardial infarction: results of the TIMI 10B trial. Thrombolysis in Myocardial Infarction (TIMI) 10B Investigators. Circulation 98(25):2805–2814
Ibanez B, James S, Agewall S et al (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39(2):119–177
Campbell BCV, Mitchell PJ, Churilov L et al (2018) Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med 378(17):1573–1582
Haley EC Jr, Thompson JL, Grotta JC et al (2010) Phase IIB/III trial of tenecteplase in acute ischemic stroke: results of a prematurely terminated randomized clinical trial. Stroke 41(4):707–711
Huang X, Cheripelli BK, Lloyd SM et al (2015) Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open-label, blinded endpoint study. Lancet Neurol 14(4):368–376
Psychogios K, Palaiodimou L, Katsanos AH et al (2021) Real-world comparative safety and efficacy of tenecteplase versus alteplase in acute ischemic stroke patients with large vessel occlusion. Ther Adv Neurol Disord 14:1756286420986727
Thommessen B, Næss H, Logallo N et al (2021) Tenecteplase versus alteplase after acute ischemic stroke at high age. INT J STROKE 16(3):295–299
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:65–94
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:25–35
Higgins JPT, Altman DG, Gotzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Menon BK, Buck BH, Singh N et al (2022) Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet 400(10347):161–169
Logallo N, Novotny V, Assmus J et al (2017) Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol 16(10):781–788
Kvistad CE, Næss H, Helleberg BH et al (2022) Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. Lancet Neurol 21:511–519
Parsons M, Spratt N, Bivard A et al (2012) A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med 366(12):1099–1107
Bivard A, Zhao H, Churilov L et al (2022) Comparison of tenecteplase with alteplase for the early treatment of ischaemic stroke in the Melbourne Mobile Stroke Unit (TASTE-A): a phase 2, randomised, open-label trial. Lancet Neurol 21:520–527
Li S, Pan Y, Wang Z et al (2022) Safety and efficacy of tenecteplase versus alteplase in patients with acute ischaemic stroke (TRACE): a multicentre, randomised, open label, blinded-endpoint (PROBE) controlled phase II study. Stroke Vasc Neurol 7(1):47–53
Van De WF, Adgey J, Ardissino D et al (1999) Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet 354(9180):716–722
Powers WJ, Rabinstein AA, Ackerson T et al (2019) Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50(12):e344–e418
Berge E, Whiteley W, Audebert H et al (2021) European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J 6(1):I–LXII
Seners P, Caroff J, Chausson N et al (2019) Recanalization before thrombectomy in tenecteplase vs. alteplase-treated drip-and-ship patients. J Stroke 21(1):105–107
Katsanos AH, Psychogios K, Turc G et al (2022) Off-label use of tenecteplase for the treatment of acute ischemic stroke: a systematic review and meta-analysis. JAMA Netw Open 5:e224506
Thelengana A, Radhakrishnan DM, Prasad M et al (2019) Tenecteplase versus alteplase in acute ischemic stroke: systematic review and meta-analysis. Acta Neurol Belg 119(3):359–367
Kheiri B, Osman M, Abdalla A et al (2018) Tenecteplase versus alteplase for management of acute ischemic stroke: a pairwise and network meta-analysis of randomized clinical trials. J Thromb Thrombolys 46(4):440–450
Campbell BCV, Mitchell PJ, Churilov L et al (2020) Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA TNK part 2 randomized clinical trial. JAMA 323:1257–1265
Burgos AM, Saver JL (2019) Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke meta-analysis of 5 randomized trials. Stroke 50:2156–2162
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
Not applicable
Informed consent
Not applicable
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Heng Wei and Bin Fu contributed equally to the manuscript as first author.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, H., Fu, B., Yang, C. et al. The efficacy and safety of intravenous thrombolysis with tenecteplase versus alteplase for acute ischemic stroke: a systematic review and meta-analysis. Neurol Sci 44, 3005–3015 (2023). https://doi.org/10.1007/s10072-023-06801-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06801-0