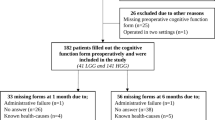
Abstract
Background
The standardization of outcome measures is needed for comparing studies and using common measures in clinical practice. We aimed to identify cognitive and patient-reported outcomes and timing of assessment for glioma, meningioma, and vascular surgery.
Method
A consensus study was conducted. Participants selected cognitive and patient-reported measures among a list of instruments identified through a literature search.
Results
Seventeen cognitive tests for the glioma and meningioma’s evaluation, 8 for the vascular diseases, and one questionnaire on quality of life and one on emotional distress were identified. The timing of outcome assessment selected was before surgery, at discharge, and after 3 and 12 months for glioma; before surgery and after 3 months for meningioma; before surgery, at discharge, and after 6 months for vascular diseases.
Conclusion
The identification of common outcome measures is the first step toward a shared data collection improving the quality and comparability of future studies.

Similar content being viewed by others
References
Kirkham JJ, Dwan KM, Altman DG et al (2010) The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 340:365. https://doi.org/10.1136/bmj.c365
Macefield RC, Jacobs M, Korfage IJ et al (2014) Developing core outcomes sets: methods for identifying and including patient-reported outcomes (PROs). Trials 15:49. https://doi.org/10.1186/1745-6215-15-49
Grinnon ST, Miller K, Marler JR et al (2012) National Institute of Neurological Disorders and Stroke Common Data Element Project-approach and methods. Clin Trials 9(3):322–329. https://doi.org/10.1177/1740774512438980
Williamson PR, Altman DG, Blazeby JM et al (2012) Developing core outcome sets for clinical trials: issues to consider. Trials 13:132. https://doi.org/10.1186/1745-6215-13-132
Papagno C, Casarotti A, Comi A et al (2012) Measuring clinical outcomes in neuro-oncology. A battery to evaluate low-grade gliomas (LGG). J Neurooncol 108(2):269–275. https://doi.org/10.1007/s11060-012-0824-5
Rofes A, Mandonnet E, Godden J et al (2017) Survey on current cognitive practices within the European Low-Grade Glioma Network: towards a European assessment protocol. Acta Neurochir (Wien) 159(7):1167–1178. https://doi.org/10.1007/s00701-017-3192-2
Yoshii Y, Tominaga D, Sugimoto K et al (2008) Cognitive function of patients with brain tumor in pre- and postoperative stage. Surg Neurol 69(1):51–61. https://doi.org/10.1016/j.surneu.2007.07.064
Dijkstra M, Van Nieuwenhuizen D, Stalpers LJA et al (2009) Late neurocognitive sequelae in patients with WHO grade I meningioma. J Neurol Neurosurg Psychiatry 80(8):910–915. https://doi.org/10.1136/jnnp.2007.138925
Waagemans ML, Van Nieuwenhuizen D, Dijkstra M et al (2011) Long-term impact of cognitive deficits and epilepsy on quality of life in patients with low-grade meningiomas. Neurosurgery 69(1):72–78. https://doi.org/10.1227/NEU.0b013e318212badb
Stienen MN, Visser-Meily JM, Schweizer TA et al (2019) Prioritization and timing of outcomes and endpoints after aneurysmal subarachnoid hemorrhage in clinical trials and observational studies: proposal of a multidisciplinary research group. Neurocrit Care 30:102–113. https://doi.org/10.1007/s12028-019-00737-0
Kazim SF, Ogulnick JV, Robinson MB et al (2021) Cognitive impairment after intracerebral hemorrhage: a systematic review and meta-analysis. World Neurosurg 148:141–162. https://doi.org/10.1016/j.wneu.2021.01.026
Armstrong TS, Dirven L, Arons D et al (2020) Glioma patient-reported outcome assessment in clinical care and research: a Response Assessment in Neuro-Oncology collaborative report. Lancet Oncol 21(2):e97–e103. https://doi.org/10.1016/S1470-2045(19)30796-X
De Witt Hamer PC, Klein M, Hervey-Jumper SL et al (2021) Functional outcomes and health-related quality of life following glioma surgery. Neurosurgery 88(4):720–732. https://doi.org/10.1093/neuros/nyaa365
Corniola MV, Meling TR (2021) Functional outcome and quality of life after meningioma surgery: a systematic review. Acta Neurol Scand 143(5):467–474. https://doi.org/10.1111/ane.13395
Van Coevorden-van Loon EMP, Heijenbrok-Kal MH, Van Loon WS et al (2015) Assessment methods and prevalence of cognitive dysfunction in patients with low-grade glioma: a systematic review. J Rehabil Med 47(6):481–488. https://doi.org/10.2340/16501977-1975
Meskal I, Gehring K, Rutten GJM, Sitskoorn MM (2016) Cognitive functioning in meningioma patients: a systematic review. J Neurooncol 128(2):195–205. https://doi.org/10.1007/s11060-016-2115-z
Andersen CR, Fitzgerald E, Delaney A, Finfer S (2019) A systematic review of outcome measures employed in aneurysmal subarachnoid hemorrhage (aSAH) clinical research. Neurocrit Care 30(3):534–541. https://doi.org/10.1007/s12028-018-0566-0
Sumsion T (1998) The Delphi technique: an adaptive research tool. Br J Occup Ther 64(4):153–156. https://doi.org/10.1177/030802269806100403
Butts AM, Weigand S, Brown PD et al (2017) Neurocognition in individuals with incidentally-identified meningioma. J Neurooncol 134:125–132. https://doi.org/10.1007/s11060-017-2495-8
Zamanipoor Najafabadi AH, Peeters MCM, Dirven L et al (2017) Impaired health-related quality of life in meningioma patients - a systematic review. Neuro Oncol 19(7):897–907. https://doi.org/10.1093/neuonc/now250
Luckett T, King MT, Butow PN et al (2011) Choosing between the EORTC QLQ-C30 and FACT-G for measuring health-related quality of life in cancer clinical research: issues, evidence and recommendations. Ann Oncol 22(10):2179–2190. https://doi.org/10.1093/annonc/mdq721
Aaronson NK, Ahmedzai S, Bergman B et al (1993) The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5):365–376. https://doi.org/10.1093/jnci/85.5.365
Costantini M, Musso M, Viterbori P et al (1999) Detecting psychological distress in cancer patients: validity of the Italian version of the Hospital Anxiety and Depression Scale. Support Care Cancer 7(3):121–127. https://doi.org/10.1007/s005200050241
Langsdale T (1997) Eq-5D: a generic tool for outcomes measurement. PharmacoEcon Outcome News 109(1):3–4. https://doi.org/10.1007/bf03271524
Waggoner J, Carline JD, Durning SJ (2016) Is there a consensus on consensus methodology? Descriptions and recommendations for future consensus research. Acad Med 91(5):663–668. https://doi.org/10.1097/ACM.0000000000001092
Schiavolin S, Raggi A, Scaratti C et al (2018) Patients’ reported outcome measures and clinical scales in brain tumor surgery: results from a prospective cohort study. Acta Neurochir (Wien) 160(5):1053–1061. https://doi.org/10.1007/s00701-018-3505-0
Sagberg LM, Drewes C, Jakola AS, Solheim O (2017) Accuracy of operating neurosurgeons’ prediction of functional levels after intracranial tumor surgery. J Neurosurg 126(4):1173–1180. https://doi.org/10.3171/2016.3.JNS152927
Reponen E, Tuominen H, Hernesniemi J, Korja M (2015) Patient-reported outcomes in elective cranial neurosurgery. World Neurosurg 84(6):1845–1851. https://doi.org/10.1016/j.wneu.2015.08.007
Drewes C, Sagberg ML, Jakola AS et al (2015) Morbidity after intracranial tumor surgery: sensitivity and specificity of retrospective review of medical records compared with patient-reported outcomes at 30 days. J Neurosurg 123:972–977. https://doi.org/10.3171/2015.3.JNS142959
Acknowledgements
The authors thank all the participants for their participation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
None.
Conflict of interest
All authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schiavolin, S., Mariniello, A., Broggi, M. et al. Patient-reported outcome and cognitive measures to be used in vascular and brain tumor surgery: proposal for a minimum set. Neurol Sci 43, 5143–5151 (2022). https://doi.org/10.1007/s10072-022-06162-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06162-0