Abstract
Background
Alzheimer’s disease (AD) diagnosis can be hindered by amyloid biomarkers discordances.
Objective
We aim to interpret discordances between amyloid positron emission tomography (Amy-PET) and cerebrospinal fluid (CSF) (Aβ42 and Aβ42/40), using Amy-PET semiquantitative analysis, [18F]fluorodeoxyglucose (FDG)-PET pattern, and CSF assays.
Method
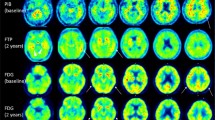
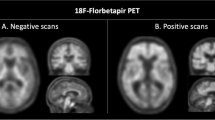
Thirty-six subjects with dementia or mild cognitive impairment, assessed by neuropsychological tests, structural and functional imaging, and CSF assays (Aβ42, Aβ42/40, p-tau, t-tau), were retrospectively examined. Amy-PET and FDG-PET scans were analyzed by visual assessment and voxel-based analysis. SUVR were calculated on Amy-PET scans.
Results
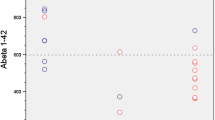
Groups were defined basing on the agreement among CSF Aβ42 (A), CSF Aβ42/40 Ratio (R), and Amy-PET (P) dichotomic results ( ±). In discordant groups, CSF assays, Amy-PET semiquantification, and FDG-PET patterns supported the diagnosis suggested by any two agreeing amyloid biomarkers. In groups with discordant CSF Aβ42, the ratio always agrees with Amy-PET results, solving both false-negative and false-positive Aβ42 results, with Aβ42 levels close to the cut-off in A + R-P- subjects. The A + R + P- group presented high amyloid deposition in relevant areas, such as precuneus, posterior cingulate cortex (PCC) and dorsolateral frontal inferior cortex at semiquantitative analysis.
Conclusion
The amyloid discordant cases could be overcome by combining CSF Aβ42, CSF ratio, and Amy-PET results. The concordance of any 2 out of the 3 biomarkers seems to reveal the remaining one as a false result. A cut-off point review could avoid CSF Aβ42 false-negative results. The regional semiquantitative Amy-PET analysis in AD areas, such as precuneus and PCC, could increase the accuracy in AD diagnosis.




Similar content being viewed by others
References
Jack CR, Bennett DA, Blennow K et al (2018) NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14(4):535–562
De Wilde A, Reimand J, Teunissen CE et al (2019) Discordant amyloid-β PET and CSF biomarkers and its clinical consequences. Alzheimers Res Ther 11(1):78 (Published 2019 Sep 12)
Blennow K, de Leon MJ, Zetterberg H (2006) Alzheimer’s disease. Lancet 368(9533):387–403
Palmqvist S, Mattsson N, Hansson O (2016) Cerebrospinal fluid analysis detects cerebral amyloid-β accumulation earlier than positron emission tomography. Brain 139(Pt 4):1226–1236
Clark CM, Pontecorvo MJ, Beach TG et al (2012) Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012 Aug;11(8):658]. Lancet Neurol 11(8):669–678
Sperling RA, Aisen PS, Beckett LA et al (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3):280–292
Dubois B, Feldman HH, Jacova C et al (2014) Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 13(6):614–629 [published correction appears in Lancet Neurol. 2014 Aug;13(8):757]
Albert MS, DeKosky ST, Dickson D et al (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3):270–279
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7(3):263–269
Blennow K, Mattsson N, Schöll M, Hansson O, Zetterberg H (2015) Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci 36(5):297–309
Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P (2019) Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimers Res Ther 11(1):34 (Published 2019 Apr 22)
Mattsson N, Insel PS, Landau S et al (2014) Diagnostic accuracy of CSF Ab42 and florbetapir PET for Alzheimer’s disease. Ann Clin Transl Neurol 1(8):534–543
Landau SM, Lu M, Joshi AD et al (2013) Comparing positron emission tomography imaging and cerebrospinal fluid measurements of beta-amyloid. AnnNeurol 74(6):826–836
Mattsson N, Insel PS, Donohue M et al (2015) Independent information from cerebrospinal fluid amyloid-β and florbetapir imaging in Alzheimer’s disease. Brain 138(Pt 3):772–783
Wiltfang J, Esselmann H, Bibl M et al (2007) Amyloid β peptide ratio 42/40 but not Aβ42 correlates with phospho-Tau in patients with low- and high-CSF Aβ40 load. J Neurochem 101(4):1053–1059
Janelidze S, Zetterberg H, Mattsson N et al (2016) J CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol 3(3):154–165
Lewczuk P, Lelental N, Spitzer P, Maler JM, Kornhuber J (2015) Amyloid-β 42/40 cerebrospinal fluid concentration ratio in the diagnostics of Alzheimer’s disease: validation of two novel assays. J Alzheimers Dis 43(1):183–191
Giacomucci G, Mazzeo S, Bagnoli S et al (2021) Matching clinical diagnosis and amyloid biomarkers in alzheimer’s disease and frontotemporal dementia. J Pers Med 11(1):47
Augutis K, Axelsson M, Portelius E et al (2013) Cerebrospinal fluid biomarkers of β-amyloid metabolism in multiple sclerosis. Mult Scler 19(5):543–552
Zwan M, van Harten A, Ossenkoppele R et al (2014) Concordance between cerebrospinal fluid biomarkers and [11C]PIB PET in a memory clinic cohort. J Alzheimers Dis 41(3):801–807
Alongi P, Sardina DS, Coppola R et al (2019) 18F-Florbetaben PET/CT to Assess Alzheimer’s Disease: a new analysis method for regional amyloid quantification. J Neuroimaging 29(3):383–393
Meyer PF, McSweeney M, Gonneaud J, Villeneuve S (2019) AD molecular: PET amyloid imaging across the Alzheimer’s disease spectrum: from disease mechanisms to prevention. Prog Mol Biol Transl Sci 165:63–106
Lombardi G, Pupi A, Bessi V et al (2020) Challenges in Alzheimer’s disease diagnostic work-up: amyloid biomarker incongruences. J Alzheimers Dis 77(1):203–217
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149(2):351–356
Rudilosso S, San Román L, Blasco J, Hernández-Pérez M, Urra X, Chamorro Á (2017) Evaluation of white matter hypodensities on computed tomography in stroke patients using the Fazekas score. Clin Imaging 46:24–27
Kollhoff AL, Howell JC, Hu WT (2018) Automation vs experience: measuring Alzheimer’s beta-amyloid 1–42 peptide in the CSF. Front Aging Neurosci 10:253 (Published 2018 Aug 22)
Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ (1994) Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2(4):189–210
Della Rosa PA, Cerami C, Gallivanone F et al (2014) A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics 12(4):575–593
Perani D, Della Rosa PA, Cerami C et al (2014) Validation of an optimized SPM procedure for FDG-PET in dementia diagnosis in a clinical setting. NeuroImage Clin 2014(6):445–454
Rolls ET, Huang CC, Lin CP, Feng J, Joliot M (2020) Automated anatomical labelling atlas 3. Neuroimage. 206:116189
Palmqvist S, Zetterberg H, Mattsson N et al (2015) Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology 85(14):1240–1249
Nobili F, Arbizu J, Bouwman F et al (2018) European Association of Nuclear Medicine and European Academy of Neurology recommendations for the use of brain 18 F-fluorodeoxyglucose positron emission tomography in neurodegenerative cognitive impairment and dementia: Delphi consensus. Eur J Neurol 25(10):1201–1217
Fodero-Tavoletti MT, Cappai R, McLean CA et al (2009) Amyloid imaging in Alzheimer’s disease and other dementias. Brain Imaging Behav 3(3):246–261
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82(4):239–259
Palmqvist S, Schöll M, Strandberg O et al (2017) Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat Commun 8(1):1214
Berti V, Pupi A, Mosconi L (2011) PET/CT in diagnosis of dementia. Ann N Y Acad Sci 1228:81–92
Nelson PT, Abner EL, Schmitt FA et al (2009) Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from alzheimer disease. J Neuropathol Exp Neurol 68(7):774–784
Weigand AJ, Bangen KJ, Thomas KR et al (2020) Is tau in the absence of amyloid on the Alzheimer’s continuum?: A study of discordant PET positivity. Brain Commun 2(1):fcz046
Weise D, Tiepolt S, Awissus C et al (2015) Critical Comparison of Different Biomarkers for Alzheimer’s Disease in a Clinical Setting. J Alzheimers Dis 48(2):425–432
Herholz K, Salmon E, Perani D et al (2002) Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage 17(1):302–316
Karas G, Scheltens P, Rombouts S et al (2007) Precuneus atrophy in early-onset Alzheimer’s disease: A morphometric structural MRI study. Neuroradiology 49(12):967–976
Acknowledgements
Thanks to Fondazione Turati for providing materials for cerebrospinal fluid assay.
Author information
Authors and Affiliations
Contributions
Matilde Nerattini: Conceptualization (supporting); writing, original draft preparation (equal); formal analysis (equal); investigation (equal).
Federica Rubino: Conceptualization (supporting); writing, original draft preparation (equal); formal analysis (equal); investigation (equal).
Annachiara ArnoneL Conceptualization (supporting); writing, original draft preparation (equal); formal analysis (equal); investigation (equal).
Cristina Polito: Methodology (equal); writing, review and editing (supporting).
Giulia Puccini: Methodology (equal); writing, review and editing (supporting).
Salvatore Mazzeo: Resources; writing, review and editing (supporting).
Gemma Lombardi: Resources; writing, review and editing (supporting).
Benedetta Nacmias: Resources; writing, review and editing (supporting).
Maria Teresa De Cristofaro: Resources; writing, review and editing (supporting).
Sandro Sorbi: Resources; supervision (supporting); writing, review and editing (supporting).
Alberto Pupi: Writing, review and editing (supporting); supervision (supporting).
Roberto Sciagrà: Writing, review and editing (supporting); supervision (supporting).
Valentina Bessi: Resources; writing, review and editing (supporting).
Valentina Berti: Conceptualization (lead); supervision (lead); writing, original draft (supporting); writing, review and editing (lead).
Corresponding author
Ethics declarations
Ethics approval
Participants (or their caregivers) gave informed consent for all investigations, and study procedures were approved by the local Institutional Review Board (reference 15691_oss).
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nerattini, M., Rubino, F., Arnone, A. et al. Cerebral amyloid load determination in a clinical setting: interpretation of amyloid biomarker discordances aided by tau and neurodegeneration measurements. Neurol Sci 43, 2469–2480 (2022). https://doi.org/10.1007/s10072-021-05704-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05704-2