Abstract
Introduction and Aim
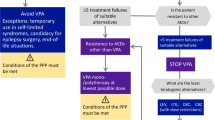
Valproic acid (Na valproate) is a broad-spectrum anti-seizure medication used in children and adolescents. It is thought to have fewer adverse effects; however, recent studies have restricted its use in women of reproductive age due to the teratogenic impacts on cognition. Although alternative drugs have been used to treat patients in clinical follow-up, some patients have to return to using valproic acid. Our study aimed to determine the rate of return to valproic acid treatment in female patients with follow-up in our centre and the reasons for the return.
Materials and Methods
Female patients with genetic generalized epilepsy who were followed up in our centre were included in the study. Patient data were retrospectively obtained from file records. The patients were grouped by seizure subgroups, antiepileptic treatment used, electroencephalography characteristics, and seizure treatment response.
Results
Sixty-three (31.7%) of the 199 patients had to return to VPA treatment. When the reasons for the discontinuation of other drugs were examined, non-response to treatment was found in 80.0% of patients, adverse medication effects in 18.3%, and 1.7% continued voluntarily. Patients who are JAE subtypes were more likely to return to VPA treatment than GTCS alone subtypes. A total of 7.4% of patients converted to VPA therapy had continued myoclonic seizures compared with 20.4% of patients treated with alternative drugs.
Conclusion
VPA treatment is not used as the first choice in females of reproductive age; however, some patients will only achieve seizure control with valproate, especially those with myoclonic seizures and JAE.

Similar content being viewed by others
References
Davis R, Peters DH, McTavish D (1994) Valproic acid. A reappraisal of its pharmacological properties and clinical efficacy in epilepsy. Drugs. 47:332–372
Eriksson K, Viinikainen K, Mönkkönen A, Äikiä M, Nieminen P, Heinonen S, Kälviäinen R (2005) Children exposed to valproate in utero: population-based evaluation of risks and confounding factors for long-term neurocognitive development. Epilepsy Res 65(3):189–200
Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Sabers A, Perucca E, Vajda F, EURAP study group (2011) Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol 10(7):609–617
Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW, NEAD Study Group (2009) Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med 360:1597–1605
Meador KJ, Baker GA, Browning N, Cohen MJ, Bromley RL, Clayton-Smith J, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW, NEAD Study Group (2013) Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol 12(3):244–252. https://doi.org/10.1016/S1474-4422(12)70323-X
Baker GA, Bromley RL, Briggs M, Cheyne CP, Cohen MJ, Garcia-Finana M, Gummery A, Kneen R, Loring DW, Mawer G, Meador KJ, Shallcross R, Clayton-Smith J, On behalf of the Liverpool and Manchester Neurodevelopment Group (2015) IQ at 6 years after in utero exposure to antiepileptic drugs: a controlled cohort study. Neurology 84(4):382–390
Shallcross R, Bromley RL, Irwin B, Bonnett LJ, Morrow J, Baker GA, Liverpool Manchester Neurodevelopment Group, UK Epilepsy and Pregnancy Register (2011) Child development following in utero exposure: levetiracetam vs sodium valproate. Neurology 76(4):383–389
Shallcross R, Bromley RL, Cheyne CP, Garcia-Finana M, Irwin B, Morrow J et al (2014) In utero exposure to levetiracetam vs valproate: development and language at 3 years of age. Neurology 82(3):213–221
Christensen J, Gronborg TK, Sorensen MJ et al (2013) Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 309:1696–1703
Bromley RL, Calderbank R, Cheyne CP, Rooney C, Trayner P, Clayton-Smith J, García-Fiñana M, Irwin B, Morrow JI, Shallcross R, Baker GA, UK Epilepsy and Pregnancy Register (2016) Cognition in school-age children exposed to levetiracetam, topiramate, or sodium valproate. Neurology 87:1943–1953
Rihtman T, Parush S, Ornoy A (2012) Preliminary findings of the developmental effects of in utero exposure to topiramate. Reprod Toxicol 34(3):308–311
IE Scheffer et al ILAE Classification of the Epilepsies Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017; 58(4): 512-521).
Epilepsies: diagnosis and management Clinical guideline [CG137] Published date: 11 January 2012 Last updated: 11 February 2020.
Seneviratne U, Hepworth G, Cook M, D'Souza W (2016) Atypical EEG abnormalities in genetic generalized epilepsies. Clin Neurophysiol 127(1):214–220
Bosak M, Słowik A, Turaj W (2019) Why do some women with epilepsy use valproic acid despite current guidelines? A single-center cohort study. Epilepsy Behav 98:1–5
Posner EB, Mohamed K, Marson AG (2005) Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents. Cochrane Database Syst Rev 4:CD003032
Prasad A, Kuzniecky RI, Knowlton RC, Welty TE, Martin RC, Mendez M, Faught RE (2003) Evolving antiepileptic drug treatment in juvenile myoclonic epilepsy. Arch Neurol 60(8):1100–1105
de Almeida Campos MS et al (2018) Comparative efficacy of antiepileptic drugs for patients with generalized epileptic seizures: systematic review and network meta-analyses. Int J Clin Pharm 40(3):589–598
Striano P, Sofia V, Capovilla G, Rubboli G, di Bonaventura C, Coppola A, Vitale G, Fontanillas L, Giallonardo AT, Biondi R, Romeo A, Viri M, Zara F, Striano S (2008) A pilot trial of levetiracetam in eyelid myoclonia with absences (Jeavons syndrome). Epilepsia. 49(3):425–430
Kim HL, Aldridge J, Rho JM (2005) Clinical experience with zonisamide monotherapy and adjunctive therapy in children with epilepsy at a tertiary care referral center. J Child Neurol 20(3):212–219
Berkovic SF, Knowlton RC, Leroy RF, Schiemann J, Falter U, On behalf of the Levetiracetam N01057 Study Group (2007) On behalf of the Levetiracetam N01057 Study Group. Placebo-controlled study of levetiracetam in idiopathic generalized epilepsy. Neurology. 69(18):1751–1760
Noachtar S, Andermann E, Meyvisch P, Andermann F, Gough WB, Schiemann-Delgado J, For the N166 Levetiracetam Study Group (2008) Levetiracetam for the treatment of idiopathic generalized epilepsy with myoclonic seizures. Neurology. 70(8):607–616
Nicolson A, Appleton RE, Chadwick DW, Smith DF (2004) The relationship between treatment with valproate, lamotrigine, and topiramate and the prognosis of the idiopathic generalized epilepsies. J Neurol Neurosurg Psychiatry 75(1):75–79
Kothare SV, Valencia I, Khurana DS, Hardison H, Melvin JJ, Legido A (2004) Efficacy and tolerability of zonisamide in juvenile myoclonic epilepsy. Epileptic Disord 6(4):267–270
Morris GL, Hammer AE, Kustra RP, Messenheimer JA (2004) Lamotrigine for patients with juvenile myoclonic epilepsy following prior treatment with valproate: results of an open-label study. Epilepsy Behav 5(4):509–512
Vossler DG, Knake S, O'Brien TJ, Watanabe M, Brock M, Steiniger-Brach B, Williams P, Roebling R, SP0982 co-investigators (2020) Efficacy and safety of adjunctive lacosamide in the treatment of primary generalized tonic-clonic seizures: a double-blind, randomized, placebo-controlled trial. J Neurol Neurosurg Psychiatry 91(10):1067–1075
Cerulli Irelli E, Morano A, Cocchi E et al (2019) Doing without valproate in women of childbearing potential with Idiopathic generalized epilepsy: Implications on seizure outcome. Epilepsia. 00:1–8
Gesche J et al (2020) Patterns and prognostic markers for treatment response in generalized epilepsies. Neurology:95/18
Cerulli Irelli E, Cocchi E, Morano A, Casciato S, Fanella M, Albini M, Fisco G, Barone FA, Orlando B, Mascia A, Manfredi M, Fattouch J, Giallonardo AT, di Gennaro G, di Bonaventura C (2020) Valproate impact and sex-dependent seizure remission in patients with idiopathic generalized epilepsy. J Neurol Sci 415:116940
Tomson T, Marson A, Boon P, Canevini MP, Covanis A, Gaily E, Kälviäinen R, Trinka E (2015) Valproate in the treatment of epilepsy in girls and women of childbearing potential. Epilepsia. 56(7):1006–1019. https://doi.org/10.1111/epi.13021
Angus-Leppan H, Moghim MM, Cock H, Kinton L, Wells MS, Shankar R (2020) Valproate risk form—Surveying 215 clinicians involving 4775 encounters. Acta Neurol Scand 141:483–490
Author information
Authors and Affiliations
Contributions
All authors managed the patients, performed the analysis, wrote and reviewed the manuscript, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Ethics committee approval of Dokuz Eylul University (No.: 2020 / 17-25) was obtained.
Informed consent
None.
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mermi Dibek, D., Öztura, İ. & Baklan, B. Our reasons for converting to valproic acid treatment in female patients with genetic generalized epilepsy: a retrospective, single-centre study. Neurol Sci 43, 517–523 (2022). https://doi.org/10.1007/s10072-021-05261-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05261-8