Abstract
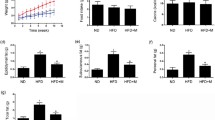
Xanthigen, a mixture of brown seaweed and pomegranate seed extracts, has weight loss properties and lipid-lowering effects in mice and humans. This study elucidated the Xanthigen mechanism of an anti-obesity activity in high-fat diet (HFD)-fed mice. Xanthigen decreased expression of peroxisome proliferator-activated receptor γ (PPARγ) in the adipose tissue of HFD-fed mice. The serum leptin level and the adipose tissue leptin expression in mice fed HFD plus Xanthigen were significantly decreased, compared to HFD-fed mice. Phosphorylation of AMPactivated protein kinase (AMPK) α and β and acetyl-CoA carboxylase (ACC) in the adipose tissue of HFD plus Xanthigen-fed mice was elevated, and HMG-CoA reductase (HMGCR) expression was decreased. Xanthigen may have an anti-obesity activity by down-regulation of PPARγ and activation of the AMPK pathway.
Similar content being viewed by others
Refrerences
Kopelman PG. Obesity as a medical problem. Nature 404: 635–643 (2000)
Eckel RH, Barouch WW, Ershow AG. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on the pathophysiologyof obesity-associated cardiovascular disease. Circulation 105: 2923–2928 (2002)
Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell 104: 531–543 (2001)
Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9: 367–377 (2008)
Tong Q, Hotamisligil GS. Molecular mechanisms of adipocyte differentiation. Rev. Endocr. Metab. Disord. 2: 349–355 (2001)
Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J. Nutr. 130: 3122S–3126S (2000)
Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol. Rev. 78: 783–809 (1998)
Dagon Y, Avraham Y, Berry EM. AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2alpha in adipocytes. Biochem. Biophy. Res. Co. 340: 43–47 (2006)
Giri S, Rattan R, Haq E, Khan M, Yasmin R, Won JS, Key L, Singh AK, Singh I. AICAR inhibits adipocyte differentiation in 3T3L1 and restores metabolic alterations in diet-induced obesity mice model. Nutr. Metab. 3: 31 (2006)
Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J. Clin. Invest. 116: 1776–1783 (2006)
Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 1: 15–25 (2005)
Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase—development of the energy sensor concept. J. Physiol. 574: 7–15 (2006)
Daval M, Foufelle F, Ferre P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 574: 55–62 (2006)
Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 546: 113–120 (2003)
Hardie DG. Minireview: The AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 144: 5179–5183 (2003)
Maeda H, Hosokawa M, Sashima T, Takahashi N, Kawada T, Miyashita K. Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int. J. Mol. Med. 18: 147–152 (2006)
Jeon SM, Kim HJ, Woo MN, Lee MK, Shin YC, Park YB, Choi MS. Fucoxanthin-rich seaweed extract suppresses body weight gain and improves lipid metabolism in high-fat-fed C57BL/6J mice. Biotechnol. J. 5: 961–969 (2010)
Vroegrijk IO, van Diepen JA, van den Berg S, Westbroek I, Keizer H, Gambelli L, Hontecillas R, Bassaganya-Riera J, Zondag GC, Romijn JA, Havekes LM, Voshol PJ. Pomegranate seed oil, a rich source of punicic acid, prevents diet-induced obesity and insulin resistance in mice. Food Chem. Toxicol. 49: 1426–1430 (2011)
Arao K, Wang YM, Inoue N, Hirata J, Cha JY, Nagao K, Yanagita T. Dietary effect of pomegranate seed oil rich in 9cis, 11trans, 13cis conjugated linolenic acid on lipid metabolism in obese, hyperlipidemic OLETF rats. Lipids Health Dis. 3: 24 (2004)
Lai CS, Tsai ML, Badmaev V, Jimenez M, Ho CT, Pan MH. Xanthigen suppresses preadipocyte differentiation and adipogenesis through down-regulation of PPARgamma and C/EBPs and modulation of SIRT-1, AMPK, and FoxO pathways. J. Agr. Food Chem. 60: 1094–1101 (2012)
Abidov M, Ramazanov Z, Seifulla R, Grachev S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 12: 72–81 (2010)
Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell 3: 127–133 (1974)
Choi KM, Lee YS, Kim W, Choi YH, Kwak YG, Jung JC, Lee J, Yoo HS. Improvement of High-fat Diet-induced Obesity by Xanthigen in C57BL/6N Mice. J. Life Sci. 22: 1697–1703 (2012)
Choi KM, Lee YS, Shin DM, Lee S, Yoo KS, Lee MK, Lee JH, Kim SY, Lee YM, Hong JT, Yun YP, Yoo HS. Green tomato extract attenuates high-fat-diet-induced obesity through activation of the AMPK pathway in C57BL/6 mice. J. Nutr. Biochem. 24: 335–342 (2013)
Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARgamma signaling and metabolism: The good, the bad and the future. Nat. Med. 19: 557–566 (2013)
Yang R, Barouch LA. Leptin signaling and obesity: Cardiovascular consequences. Circ. Res. 101: 545–559 (2007)
Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1: 1155–1161 (1995)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, KM., Jeon, Y.S., Kim, W. et al. Xanthigen attenuates high-fat diet-induced obesity through down-regulation of PPARγ and activation of the AMPK pathway. Food Sci Biotechnol 23, 931–935 (2014). https://doi.org/10.1007/s10068-014-0125-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-014-0125-1