Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory disease that carries high social and economic costs and can lead to permanent disability. RA pathogenesis has not been completely elucidated yet. Extracellular vesicles (EVs) are membrane-contained vesicles released by cells playing a role in cell-to-cell communication and they could be involved in different diseases. Evidence on the involvement of EVs in RA is currently inconclusive. Therefore, a systematic review on the role of EVs in RA was performed in order to explore this relationship. This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The research was conducted on PubMed, Scopus, and Embase up to March 5, 2020: 41 studies were analyzed out of 674 screened. The total plasmatic and synovial fluid (SF) EV number seems increased in RA as compared with healthy controls. Both RA plasma and SF contained EVs subpopulations of heterogenous origin, especially derived from platelets and immune system cells. No univocal evidence emerged on miRNA expression and EV content profile within RA patients. EVs showed to enhance pro-inflammatory pathways, such as cytokines and chemokine release and TNF blockade seemed to revert this effect. Our work highlights the requirement to standardize study methodologies in order to make results comparable and draw conclusions that remain, at present, unclear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease with a considerably high social and economic impact. RA can result in loss of function, permanent disability, and severe systemic complications, such as cardiovascular events. RA affects about 0.5–1% of the general population worldwide and it involves any age group, with a predominance for the third, fourth and fifth decades [1]. RA is still considered an incurable disorder, even if disease remission can be obtained with tight control and treat-to-target strategies, as suggested by current recommendations [2]. Disease flares occur in more than half of the patients and they substantially contribute to radiographic damage, poorer quality of life, disability, healthcare use, and costs [3, 4]. Presently, there are no predictors of therapy response and no indications about personalized treatment.
Despite recent advances, RA pathogenesis has not been completely elucidated yet. The genetic background plays a relevant role in RA susceptibility, but its contribution to the pathogenesis is partial [5]. Besides this, environmental factors, such as cigarette smoke and air pollution, have been identified as potential triggers for RA [6,7,8].
Extracellular vesicles (EVs) are membrane-contained vesicles released by cells in all biological fluids and they have been described as impaired in many pathological conditions, such as RA. EVs can transmit molecular effectors to other cells, thereby affecting the recipient cell function. EVs can be classified into three main groups: microvesicles, formed by external budding and fission of the plasma membrane; exosomes, produced within the cell and set loose after fusion of vesicular bodies with the plasma membrane; apoptotic bodies, released like blebs of apoptotic cells [9]. Studies on EVs made use of different terminology to describe EVs and this could pose difficulties for a direct comparison between them. For this reason, in our review, according to the last version of the position statement of the International Society for Extracellular Vesicles [10], we use the term EVs as a generic term to include the whole group of EVs.
Plasmatic EVs have been proposed as potential biomarkers. Furthermore, it has been suggested that EVs, after internalization into target cells through surface-expressed ligands, may transfer miRNAs, enabling inter-cellular and inter-organ communication [11]. To our knowledge, although several reviews have been published so far, none included a full systematic revision of the literature about EVs in RA [12,13,14,15].
It was against this background that we sought to explore the role of EVs in RA to present a complete and comprehensive recap about the state of the art on this topic.
Materials and methods
Protocol and registration
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting systematic reviews and meta-analyses [16, 17]. Our protocol was registered on PROSPERO (CRD42020181164) [18].
Literature search
A comprehensive systematic literature search was undertaken using PubMed, Embase, and Scopus. The search strategy was planned to capture all the studies focusing on EVs in patients with RA with no restrictions for sex or therapies. The search terms were adapted according to bibliographic databases in combination with database-specific filters, where these were available. The full search strategy is detailed in supplementary materials (table S1).
Eligibility criteria
Inclusion criteria: studies including adult patients with RA and investigating EVs, regardless of the technique applied. Clinical trials, observational studies (cross-sectional, prospective, and retrospective), case series (if subjects were ≥ 5), studies with at least an abstract in English, and studies published from database inception to March 2020 were included.
Exclusion criteria: studies on patients under 18 years of age, review articles, animal or cell models, case reports or case series with ≤ 5 subjects, and editorials were excluded.
Data extraction and synthesis
The research was performed on 5 March 2020. The citations were imported into the reference management software package Endnote X8. Duplicated references were automatically eliminated both by the Endnote software and manually by two reviewers (T.U. and T.S.).
During the first screening, the two reviewers independently screened titles/abstracts from the list of records retrieved, and full papers were sought when abstracts were felt to be relevant. Moreover, reference lists of the reviewed articles were examined for relevant studies. In cases of disagreement, a decision was made by consensus.
The two investigators, then, independently analyzed the full-text papers and extracted the relevant data from the included studies in standardized data extraction forms. The two authors then crosschecked the extracted data to rule out any discrepancies. Unresolved disagreements between two reviewers were resolved by consensus. The following data were evaluated: first author's surname, publication year, country, study design, setting, diagnostic criteria, outcomes measured, patient enrolment strategies, methods for EVs analysis, participant characteristics (age, gender, therapies), and results.
Results
Literature review
In total, 674 references were retrieved in the initial search strategy in Medline via PubMed, Embase, and Scopus. 333 references were excluded as duplicates. 268 references were excluded after title/abstract screening. Exclusion reasons were: studies not considering EVs (n = 61), basic science studies (n = 40), studies not considering RA (n = 102), type of articles (editorial/letter/comment/book; n = 55), case reports (n = 8), duplicated (n = 2). 73 articles were retrieved for full paper review of which 41 references fulfilled the inclusion criteria. Thirty-two manuscripts were excluded (exclusion reasons are reported in Fig. 1). The review flow process is outlined in Fig. 1. All the findings of the studies included in the present systematic review are reported in Table 1 and supplementary materials (table S2).
Regarding the methods used for EV analysis, the vast majority of the studies used flow cytometry specifying the different cluster of domain (CD); more information about the methods can be found in supplementary materials (table S2).
EV concentration
Total plasmatic EV number was higher in RA than healthy controls (HC), as reported in 4 studies for a total of more than 180 patients [19, 23, 51, 54]. However, in 3 studies for a total of 74 RA patients, the EV concentration was found similar between RA and HC [20, 24, 32]. Due to differences in techniques used for EV concentration, a meta-analysis could not be performed. Moreover, the EV number did not seem a specific biomarker as it was found similar in patients with reactive arthritis (ReA) [21], undifferentiated arthritis (UA) [55], systemic lupus erythematosus (SLE) [54], primary Sjögren syndrome (pSS) [54], and osteoarthritis (OA) [21, 22].
According to one study on 41 RA patients, total plasmatic EV concentration was not different between seronegative and seropositive RA [20]. Surprisingly, in one study on 60 RA patients, EV count was found statistically different between HC and only a subpopulation of RA seropositive patients (not for those patients positive for rheumatoid factor—RF, and anti-citrullinated peptides antibodies—ACPA, at high titer) [26].
Total EVs were higher in RA synovial fluid (SF) than HC plasma [24], OA SF [42, 51], and ReA SF [42]. RA SF EVs were at the same level when compared with RA plasma [24]. According to a different study, total EVs were higher in RA SF than in RA plasma [46].
EV size
EVs from RA patients were heterogeneous in size, mostly 100–300 nm and 700–3000 nm [33]. According to a different study, smaller EVs (100 ± 50 nm) mostly derived from platelets (CD42+), while larger EVs (100–1000 nm) were more of B (CD19+) and T cells (CD3+) origin [36].
Plasmatic EV size was not different in RA as compared with HC [20, 38]. Moreover, plasmatic EV size was found similar between seronegative and seropositive RA [20]. Another study found that RA seropositive for RF and ACPA (but not if seropositivity was at high titer) had a decreased proportion of 0.1–1 μm and an elevated proportion of 1–3 μm and 3–6 μm EVs, when compared with HC [26]. Moreover, EVs from seropositive individuals had higher frequencies and wider distribution of IgM+ and IgG+ EVs [26].
Of note, some of the aforementioned studies on EV dimensions could arise concerns about the methodology used (e.g., aggregation of EVs could not have been considered). Moreover, methods reporting EVs size are often not compliant with the latest recommendations [10] and this makes comparison across different studies insidious.
EV cell origin
Many of the studies included in this systematic review focused on surface molecules with the intent of unraveling EV origin and function. Therefore, various aspects were studied: platelets (CD41, CD42a, CD62P), leukocytes (CD45), T and B lymphocytes (CD3, CD4, CD8, CD20, CD154), granulocytes and monocytes (CD14, CD16, CD66b), endothelial cells (CD146, CD62P), and cell adhesion markers (CD31, CD61).
CD3+/HLA-DR+ [48], CD3+/CD4+ [48], CD146+ [19], CD66b+ [19], CD31+ [19, 23], CD41+ [37], CD42a [41], CD61+ [34, 41, 51, 54], and CD45+ [29] plasmatic EVs were found increased in RA when compared with HC. CD41+ [23], CD45+ [23, 54], CD66b+ [41], CD62P+ [49], CD154 [49], and CD16+ [41] plasmatic EVs were found similar between RA and HC. Of note, in a study on 55 RA patients, CD14+, CD41+, CD62E+, and CD20+ EVs were higher only in patients with high disease activity as compared with HC [57].
On the contrary, according to a different study, CD20+, CD4+, CD8+, CD14+, CD66b+ plasmatic EVs were not detectable in RA, as well as in OA patients [29]. CD45+ and CD61+ EVs were found at higher levels in RA than OA [29], whereas no difference was found for CD3+/HLA-DR+, CD3+/CD4+, CD3+/CD8+ EVs between RA and OA [48]. Moreover, RA patients showed lower levels of CD45+ EVs than pSS [54] and of CD3+/CD8+ plasmatic EVs, when compared with EBV infection [48].
In RA SF, CD4+ [55], CD41+ [25], CD66+ [28, 55] and CD14+ [28, 55] EVs were found abundant, whereas glycophorin A [28, 55], CD4+ [28], CD61+ [28, 55], CD8+ [28, 55], and CD20+ [28, 55] EVs were low. These differences were not specific for RA, since they were also found in non-RA arthritis SF [28].
RA SF contained more CD61+ [51], CD45+ [29], CD3+ [39], CD4+ [29, 40], CD4+/CD161+/CD39+ [40], CD4+/CD73+/CD39+ [40], CD8+ [29, 39], CD14+ [29], and CD66+ [29] EVs than OA SF, whereas there was no difference for CD20+ EVs [29]. Annexin A1+, CD66b+, CD14+, and CD3+ EVs were higher in RA SF than RA plasma [46]. SF CD66b+ EVs were more abundant than CD14+ and CD3+ EVs [46], whereas CD3+ and CD8+ EVs were absent in plasma [39].
EV content
More citrullinated peptides and IgG were found in EVs, when compared with HC, in a study on 18 RA patients [49]. Citrullinated and not-citrullinated proteins were also present in RA, OA, and ReA, but fibronectin/IgG immunocomplexes (ICs) were found only in RA EVs [21]. According to another study, platelet EVs contained citrullinated epitopes, which were recognized by ACPA (vimentin and fibrinogen) [33]. Plasmatic EV protein content was not different in seronegative and seropositive RA [20]. IgM-RF was found in EVs in about half of RA patients seropositive for RF [20]. Burbano C. et al. found differences according to seropositivity: IC-EVs were higher in seropositive patients, whereas there was no difference in seronegative patients, as compared with HC [26]. Moreover, a similar difference between seronegative and seropositive patients was observed concerning systemic inflammation and EVs positive for IgG, IgM, CD41, and citrulline [26]. Furthermore, there were more IC-EVs in RA patients than in psoriatic arthritis (PsA) [33]. EVs with IgM and IgG were higher in RA SF than RA and HC plasma [24].
EVs with C1q, C3, and C4 were higher in RA SF than HC and RA plasma, while no differences were found between RA and HC plasma [24]. On the contrary, plasmatic EVs with C1q were higher when compared with HC in a study on 24 RA patients [32].
EVs with C-reactive protein (CRP) and serum amyloid P (SAP) were not different between RA and HC [24], whereas EVs with CRP and SAP were higher, as compared with HC in another study [32]. Serum and EV amyloid A (AA) levels were higher in patients with active disease than in patients in clinical remission [58]. Moreover, serum and EVs AA levels correlated with each other [58].
We found 6 studies that reported results on EVs miRNA. miR-150-5p expression was lower in RA than OA [30]. miR-6089, miR-6891-3p, and miR-548-3p were decreased in the serum of RA as compared with HC [35, 44]. miR-17, miR-19b, and miR-121 were overexpressed in RA [31]. Moreover, miR-6089 was found to negatively correlate with CRP, RF, and ESR [35]. Fan W. et al. found that 36 miRNAs (see Table 1 for details) were differently regulated in RA compared with HC [47]. In this study, 5 miRNAs (hsa-miR-151a-3p, hsa-miR-199a-5p, hsa-miR-370-3p, hsa-miR-589-5p, and hsa-miR-769-5p) were present in different forms of inflammatory arthritis (PsA, RA and gout) [47]. Moreover, 12 miRNAs, linked to programmed death (PD)-1/PD-ligands, were identified [38].
Thrombin-generating capacity (factor VIIa) was higher for SF EVs than plasma from RA patients and HC. No tissue factor (TF) antigen was present on SF EVs despite they were able to initiate TF-mediated thrombin generation [28]. C-type lectin-like receptor 2 (CLEC-2) on CD41+ EVs were similar, whereas GPIV on CD41+ were higher in RA than HC [37].
In RA patients, plasmatic EVs were found strongly bound to annexin V [22] and at a higher concentration than HC [29] and PsA [33]. This was not confirmed by a different study, since Annexin V+ EVs did not differ between RA and HC [49]. RA SF EVs boundless annexin V as compared with plasmatic EVs [28] and their number was not significantly elevated, as compared with OA [39]. Moreover, RA SF contained more annexin V+ [29] EVs than OA SF.
Furthermore, evidence from single studies suggested a potential role for PD-1 [38], TNF-α [59], RANK [36], TLR4 [44], and TLR3 [56].
EV biological effect
Many studies focused on EV effects on pathways related to inflammation. Plasmatic EVs and IC-EVs enhanced adhesion molecules (ICAM-1, ICAM-2), inflammatory cytokines (IL-6, IL-8), and chemokines (CCL-2, CCL-5) from endothelial cells increasing vascular permeability [22] and leukotriene release from neutrophils [33].
Plasmatic EVs from seropositive patients [26] and plasmatic EVs from patients with high disease activity [57] stimulated mononuclear phagocytes to release pro-inflammatory cytokines: TNFα [26, 57], IL-6 [26], IL-17 [57], and IL-1 [26, 57].
RA fibroblast-like synoviocytes (FLS) induced a decrease in GM-CSF [55] and an increase in MCP-1 [55], sICAM-1 [55], VEGF [55], RANTES [55], BAFF [42], IL-6 [42, 55], and IL-8 [42, 55] after stimulation with SF EVs. In one of the two studies, this ability was independent from EV origin (OA or RA) [42].
EVs from Jurkat cells stimulated the induction of several chemokines (CXCL1, CXCL2, CXCL3x, CXCL5, and CXCL6) [50] in RA synovial fibroblasts. Moreover, EVs in RA synovial fibroblasts were demonstrated to play a role in MMP-1, IFN-γ, and IL-2 secretion [59]: as expected, these biological effects were partially reduced with the exposure of TNF inhibitors [59]. RA synovial fibroblasts EVs promoted NFkB signaling pathway in both RA and OA synovial fibroblasts [59]. RA synovial fibroblasts incubated with EVs from HC produced dose-dependently PGE2 regardless of EVs origin without increasing phospholipase A2 [43]. EVs dose-dependently induced COX-2 and mPGES-1 mRNA in RA synovial fibroblasts, but not COX-1, mPGES-2, and cytosolic PGES [43]. Moreover, EVs were also able to transfer arachidonic acid from leukocytes into synovial fibroblasts [43]. Levels of total and platelet plasmatic EVs were inversely correlated with secretory phospholipase A2 (sPLA2) activity in one study [54].
SF CD161+/CD39+ EVs increased CCL20 production, SF CD73+/CD39+ EVs increased CCL17 and CCL22 synthesis in RA fibroblasts, whereas SF CD161+/CD39+ EVs increased IL-17 production, and SF CD39+/CD73+ EVs reduced IL-17 and increased IL-10 production in PBMC in RA [40].
EVs were also found involved in intracellular pro-inflammatory pathways, in particular NFkB. EVs from HC activated NF-kB and AP-1 signaling in RA synovial fibroblasts and they increased p38 and JNK, but only the inhibition of JNK caused a significant reduction in PGE2 production [43].
RA IC-EVs promoted more macrophages differentiation towards a pro-inflammatory profile (M1-like) than HC [27]. Macrophages differentiated with RA IC-EVs were resistant to repolarization to M2-like after treatment with IL-4 [27]. Macrophages were also able to enhance T and B cells and prevent B cell death [27]. Plasmatic RA EVs inhibited T-reg, possibly through miRNAs (miR-17) [31].
Plasmatic CD14+ and CD41+ EVs from RA patients showed an anti-angiogenic effect, while CD62E+ and CD144+ EVs promoted endothelial activation [19]. RA plasmatic EVs increased dose-dependently apoptosis and autophagy of endothelial cells [23]. In vitro evidence suggested that SF EVs were able to induce ECs migration without affecting ECs proliferation or viability [50]. Exo150 downregulated the expression of MMP14 and VEGF in RA FLS and inhibited migration and angiogenesis in vitro [30].
One study provided interesting but non-conclusive insights about the role of exofacial thiol EVs and oxidative stress resistance that could also play a role in RA [45].
EVs and disease characteristics
Plasmatic CD146+ EVs [19] and SF CD41+ EVs [39] levels correlated with disease duration. SF CD66b+ EVs were more abundant in established RA than early RA [29], and they were associated with the age at diagnosis [19].
A positive association was found between RF and plasmatic CD14+ [19], SF CD3+ [39], SF CD4+ [39], SF CD8+ [39], and SF CD4+/CD161+/CD39+ EVs [40]. Conversely, a negative association was observed for RF and SF CD73+/CD39+ EVs [40]. Seropositive RA patients were found to have more CD41+ EVs [26], whereas seronegative had more CD105+ EVs [26]. On the contrary, according to a different study, there was no difference regarding plasmatic EV profile between serological RA phenotypes [29]. Regarding EVs content, low levels of serum exosomal miR-548a-3p were associated with higher levels of RF [44].
SF CD3+, CD4+, and CD8+ EVs did not correlate with ACPA [39]. On the other side, ACPA positive patients had greater levels of SF annexin V+/CD45+ EVs. Moreover, a weak correlation was observed between ACPA titer, CD4+ EVs, and annexin V+/CD45+ EVs [29].
SF annexin V+ EVs and platelet-derived EVs were increased in RA patients with extra-articular symptoms [29]. Total plasmatic EV number was higher in RA than in patients with cardiovascular risk factors and their concentrations correlated with the number of traditional cardiovascular risk factors [19].
Plasmatic CD14+ and CD62E+ EVs and urinary CD14+ and CD19+ EVs differed between patients in low or high disease activity [57]. Moreover, an association was found between disease activity score on 28 joints (DAS28), plasma, and urine EVs [57].
According to another study, patients with active disease displayed an association between LYmphatic Vessel Endothelial hyaluronic acid receptor-1 (LYVE-1), both in serum and in EVs, and CRP, ACPA titer, and exosomal AA [58].
Many studies, except one [34], reported an interaction between plasmatic EVs or their content—such as miRNAs—and plasmatic inflammatory markers (ESR and CRP) [19, 20, 32, 41, 44, 58]. Four papers included tender and swollen joint count in their analysis with different outcomes: plasmatic EVs [19, 23] or SF EVs [40] influenced the joint count. Conversely, in a different study, there was no statistical correlation between plasmatic EVs and joint count [20].
One study highlighted a significant association between plasmatic EVs containing TNFα and disease activity (DAS28 or clinical disease activity index—CDAI) [23]. Several studies detected a positive correlation between DAS28 and plasmatic [19, 20, 34, 41, 57], SF [40] or urinary [57] EVs. This association was not confirmed in other four studies concerning plasmatic EV [41, 54] and SF EVs [39, 40]. Platelet EVs were similar in active and non-active RA patients [34].
Effects of therapy on EVs
Patients treated with tocilizumab displayed lower levels of plasmatic CD3+CD31+ and CD66b+ EVs, whereas patients receiving methotrexate had decreased levels of plasmatic CD3+CD31+ EVs [19]. Adversely, another study did not find any difference in plasmatic and urinary EVs levels between treated and untreated subjects [57].
Four studies reported a decrease in either plasmatic EVs [23, 53] or urinary EVs [57] after treatment with biological disease-modifying anti-rheumatic drugs (DMARDs) or leukocytapheresis [41], whereas one paper did not report an influence of conventional synthetic (cs)-DMARDs on total plasmatic EVs [32].
A decreased exo-miR-155 expression was found in patients HCV positive treated with rituximab as compared with subjects treated with anti-TNFα or csDMARDs [52].
Of note, in these studies, RA populations enrolled are extremely heterogenous (e.g. different disease activity and therapies) and, for this reason, caution is needed in drawing conclusions.
Discussion
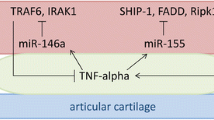
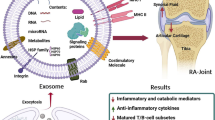
We included and reported the results of 41 studies in this systematic review. An overview of the results and a possible role of EVs in RA is reported in Fig. 2.
The total plasmatic EV concentration seems increased in RA when compared with HC, whereas plasmatic EV size was not found dissimilar between the two groups. As already highlighted, some of the studies reporting EV size might not have considered possible shortcomings of methodology. Inconsistency among the included studies could also be explained by the relatively small population enrolled. Moreover, the total plasmatic EV number was found similar in RA and other inflammatory (i.e., SLE, UA, or ReA) and non-inflammatory conditions (i.e., OA). Conversely, RA EV concentrations in SF were described at higher levels when compared with HC, OA, and ReA.
The kaleidoscopic effects of EVs on pro-inflammatory pathways, coagulation, and angiogenesis were explored in several studies. Plasmatic EVs subpopulations, based on surface molecules and linked to numerous biological effects (e.g., cell adhesion, immune system, platelet function, vascular system, and hematopoiesis), were found higher in RA than HC, even though this was not confirmed by all studies. Interestingly, in one study, similar results were found only for high disease activity RA. Moreover, different EV subtypes were found dissimilar according to disease characteristics, such as disease duration and age at diagnosis. Plasmatic EVs and IC-EVs enhanced inflammatory pathways and cytokines (e.g., IL-6); this was true especially for EVs derived from seropositive and high disease activity patients. Studies investigating whether therapies affect EVs yielded contrasting results. Nevertheless, some in vitro and in vivo evidence could suggest a possible role of inflammatory mediators, targeted by biological therapies (e.g., TNF-α and IL-6), in the pathogenesis mediated by EVs. Despite several studies found a correlation between EVs and disease activity, this could not be confirmed in other ones. The differences observed between RA and HC were less marked when RA patients were compared with OA. Moreover, some evidence suggested that EVs containing citrullinated peptides were more abundant in RA than HC but these data were not specific for RA when compared with other arthritides.
Likewise, RA SF showed more abundant EVs derived from immune cells and platelets when compared with OA, but this could not be confirmed with respect to other non-RA inflammatory arthritides.
A limited number of studies, taking into consideration miRNAs, showed that their expression was different in RA versus HC. Furthermore, some data suggested that these differences were not specific for RA since they were not dissimilar from other pathological conditions (e.g., PsA).
EVs seem to enhance the inflammatory process, but their role is not specific for RA. As expected, since RA has the joints as the main target, studies on SF and synovial fibroblasts uncovered differences more specific to RA and, consequently, studies on them are more promising.
Even though a multitude of information could be obtained, definitive conclusions about the role of EVs in RA can difficultly be drawn. Indeed, the analyzed articles varied greatly in methodology and this makes direct comparisons challenging. Moreover, some studies were conducted on small and poorly characterized (e.g., seropositivity, therapy) populations. Various narrative reviews on this topic have been published so far, but they seem to lean excessively on the positive findings partially neglecting the negative ones.
In conclusion, EVs could contribute to better understand RA pathogenesis and they could represent a possible therapeutic target [60]. Moreover, EVs could be of help in the diagnosis (inflammatory vs. non-inflammatory joint diseases), prognosis (e.g., extra-articular involvement) and therapeutic response. Studies focusing on this topic are urged to follow rigorous methodology [10], so that the scientific community can compare them and draw translatable observations.
Data availability
The whole review process has been performed according to PRISMA statements.
References
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH, Strand V, Yamamoto K (2018) Rheumatoid arthritis. Nat Rev Dis Prim 4:18001. https://doi.org/10.1038/nrdp.2018.1
Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, McInnes IB, Sepriano A, van Vollenhoven RF, de Wit M, Aletaha D, Aringer M, Askling J, Balsa A, Boers M, den Broeder AA, Buch MH, Buttgereit F, Caporali R, Cardiel MH, De Cock D, Codreanu C, Cutolo M, Edwards CJ, van Eijk-Hustings Y, Emery P, Finckh A, Gossec L, Gottenberg JE, Hetland ML, Huizinga TWJ, Koloumas M, Li Z, Mariette X, Muller-Ladner U, Mysler EF, da Silva JAP, Poor G, Pope JE, Rubbert-Roth A, Ruyssen-Witrand A, Saag KG, Strangfeld A, Takeuchi T, Voshaar M, Westhovens R, van der Heijde D (2020) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 79(6):685–699. https://doi.org/10.1136/annrheumdis-2019-216655
Bykerk VP, Shadick N, Frits M, Bingham CO 3rd, Jeffery I, Iannaccone C, Weinblatt M, Solomon DH (2014) Flares in rheumatoid arthritis: frequency and management. A report from the BRASS registry. J Rheumatol 41(2):227–234. https://doi.org/10.3899/jrheum.121521
Markusse IM, Dirven L, Gerards AH, van Groenendael JH, Ronday HK, Kerstens PJ, Lems WF, Huizinga TW, Allaart CF (2015) Disease flares in rheumatoid arthritis are associated with joint damage progression and disability: 10-year results from the BeSt study. Arthritis Res Ther 17:232. https://doi.org/10.1186/s13075-015-0730-2
MacGregor AJ, Snieder H, Rigby AS, Koskenvuo M, Kaprio J, Aho K, Silman AJ (2000) Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum 43(1):30–37. https://doi.org/10.1002/1529-0131(200001)43:1<30::AID-ANR5>3.0.CO;2-B
Hwang JY, Randall TD, Silva-Sanchez A (2016) Inducible Bronchus-Associated Lymphoid Tissue: Taming Inflammation in the Lung. Front Immunol 7:258. https://doi.org/10.3389/fimmu.2016.00258
Hart JE, Laden F, Puett RC, Costenbader KH, Karlson EW (2009) Exposure to traffic pollution and increased risk of rheumatoid arthritis. Environ Health Perspect 117(7):1065–1069. https://doi.org/10.1289/ehp.0800503
Anderson R, Meyer PW, Ally MM, Tikly M (2016) Smoking and Air Pollution as Pro-Inflammatory Triggers for the Development of Rheumatoid Arthritis. Nicotine Tob Res 18(7):1556–1565. https://doi.org/10.1093/ntr/ntw030
Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4:27066. https://doi.org/10.3402/jev.v4.27066
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borras FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MA, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Gorecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzas EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekstrom K, El Andaloussi S, Elie-Caille C, Erdbrugger U, Falcon-Perez JM, Fatima F, Fish JE, Flores-Bellver M, Forsonits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gamez-Valero A, Gardiner C, Gartner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Gorgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanovic MM, Kovacs AF, Kramer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lasser C, Laurent LC, Lavieu G, Lazaro-Ibanez E, Le Lay S, Lee MS, YXF L, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Line A, Linnemannstons K, Llorente A, Lombard CA, Lorenowicz MJ, Lorincz AM, Lotvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Moller A, Moller Jorgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O’Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Ostergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saa P, Sahoo S, Salas-Huenuleo E, Sanchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schoyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sodar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot E, Verweij FJ, Vestad B, Vinas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yanez-Mo M, Yin H, Yuana Y, Zappulli V, Zarubova J, Zekas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7(1):1535750. https://doi.org/10.1080/20013078.2018.1535750
Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10(12):1470–1476. https://doi.org/10.1038/ncb1800
Fu H, Hu D, Zhang L, Tang P (2018) Role of extracellular vesicles in rheumatoid arthritis. Mol Immunol 93:125–132. https://doi.org/10.1016/j.molimm.2017.11.016
Li Z, Wang Y, Xiao K, Xiang S, Li Z, Weng X (2018) Emerging Role of Exosomes in the Joint Diseases. Cell Physiol Biochem 47(5):2008–2017. https://doi.org/10.1159/000491469
Krajewska-Wlodarczyk M, Owczarczyk-Saczonek A, Zuber Z, Wojtkiewicz M, Wojtkiewicz J (2019) Role of Microparticles in the Pathogenesis of Inflammatory Joint Diseases. Int J Mol Sci 20(21). https://doi.org/10.3390/ijms20215453
Tavasolian F, Moghaddam AS, Rohani F, Abdollahi E, Janzamin E, Momtazi-Borojeni AA, Moallem SA, Jamialahmadi T, Sahebkar A (2020) Exosomes: Effectual players in rheumatoid arthritis. Autoimmun Rev 19(6):102511. https://doi.org/10.1016/j.autrev.2020.102511
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1. https://doi.org/10.1186/2046-4053-4-1
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151(4):W65–W94. https://doi.org/10.7326/0003-4819-151-4-200908180-00136
Schioppo T, Ubiali T, Ingegnoli F (2020) The role of extracellular vesicles in rheumatoid arthritis: a systematic review. PROSPERO: International prospective register of systematic reviews CRD42020181164
Rodríguez-Carrio J, Alperi-López M, López P, Alonso-Castro S, Carro-Esteban SR, Ballina-García FJ, Suárez A (2015) Altered profile of circulating microparticles in rheumatoid arthritis patients. Clin Sci 128(7):437–448. https://doi.org/10.1042/CS20140675
Arntz OJ, Pieters BCH, Thurlings RM, Wenink MH, van Lent PLEM, Koenders MI, van den Hoogen FHJ, van der Kraan PM, van de Loo FAJ (2018) Rheumatoid Arthritis Patients With Circulating Extracellular Vesicles Positive for IgM Rheumatoid Factor Have Higher Disease Activity. Front Immunol 9:2388. https://doi.org/10.3389/fimmu.2018.02388
Skriner K, Adolph K, Jungblut PR, Burmester GR (2006) Association of citrullinated proteins with synovial exosomes. and. Rheumatism 54(12):3809–3814. https://doi.org/10.1002/art.22276
Atehortúa L, Rojas M, Vásquez G, Muñoz-Vahos CH, Vanegas-García A, Posada-Duque RA, Castaño D (2019) Endothelial activation and injury by microparticles in patients with systemic lupus erythematosus and rheumatoid arthritis. Res Ther 21(1):34. https://doi.org/10.1186/s13075-018-1796-4
Barbati C, Vomero M, Colasanti T, Diociaiuti M, Ceccarelli F, Ferrigno S, Finucci A, Miranda F, Novelli L, Perricone C, Spinelli FR, Truglia S, Conti F, Valesini G, Alessandri C (2018) TNFα expressed on the surface of microparticles modulates endothelial cell fate in rheumatoid arthritis. Res Ther 20(1):273. https://doi.org/10.1186/s13075-018-1768-8
Biró É, Nieuwland R, Tak PP, Pronk LM, Schaap MCL, Sturk A, Hack CE (2007) Activated complement components and complement activator molecules on the surface of cell-derived microparticles in patients with rheumatoid arthritis and healthy individuals. Ann Rheum Dis 66(8):1085–1092. https://doi.org/10.1136/ard.2006.061309
Boilard E, Nigrovic PA, Larabee K, Watts GFM, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O'Donnell E, Farndale RW, Ware J, Lee DM (2010) Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327(5965):580–583. https://doi.org/10.1126/science.1181928
Burbano C, Rojas M, Muñoz-Vahos C, Vanegas-García A, Correa LA, Vásquez G, Castaño D (2018) Extracellular vesicles are associated with the systemic inflammation of patients with seropositive rheumatoid arthritis. Sci Rep 8(1):17917. https://doi.org/10.1038/s41598-018-36335-x
Burbano C, Villar-Vesga J, Vásquez G, Muñoz-Vahos C, Rojas M, Castaño D (2019) Proinflammatory differentiation of macrophages through microparticles that form immune complexes leads to T-and B-cell activation in systemic autoimmune diseases. Front Immunol 10(AUG). https://doi.org/10.3389/fimmu.2019.02058
Berckmans RJ, Nieuwland R, Tak PP, Böing AN, Romijn FPHTM, Kraan MC, Breedveld FC, Hack CE, Sturk A (2002) Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. and. Rheumatism 46(11):2857–2866. https://doi.org/10.1002/art.10587
Michael BNR, Kommoju V, Kavadichanda Ganapathy C, Negi VS (2019) Characterization of cell-derived microparticles in synovial fluid and plasma of patients with rheumatoid arthritis. Rheumatol Int 39(8):1377–1387. https://doi.org/10.1007/s00296-019-04337-1
Chen Z, Wang H, Xia Y, Yan F, Lu Y (2018) Therapeutic potential of mesenchymal cell-derived miRNA-150-5p-expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J Immunol 201(8):2472–2482. https://doi.org/10.4049/jimmunol.1800304
Wang L, Wang C, Jia X, Yu J (2018) Circulating exosomal miR-17 inhibits the induction of regulatory T cells via suppressing TGFBR II expression in rheumatoid arthritis. Physiol Biochem 50(5):1754–1763. https://doi.org/10.1159/000494793
Van Eijk IC, Tushuizen ME, Sturk A, Dijkmans BAC, Boers M, Voskuyl AE, Diamant M, Wolbink GJ, Nieuwland R, Nurmohamed MT (2010) Circulating microparticles remain associated with complement activation despite intensive anti-inflammatory therapy in early rheumatoid arthritis. Ann Rheum Dis 69(7):1378–1382. https://doi.org/10.1136/ard.2009.118372
Cloutier N, Tan S, Boudreau LH, Cramb C, Subbaiah R, Lahey L, Albert A, Shnayder R, Gobezie R, Nigrovic PA, Farndale RW, Robinson WH, Brisson A, Lee DM, Boilard E (2013) The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: The microparticle-associated immune complexes. EMBO Mol Med 5(2):235–249. https://doi.org/10.1002/emmm.201201846
Knijff-Dutmer EAJ, Koerts J, Nieuwland R, Kalsbeek-Batenburg EM, Van De Laar MAFJ (2002) Elevated levels of platelet microparticles are associated with disease activity in rheumatoid arthritis. and. Rheumatism 46(6):1498–1503. https://doi.org/10.1002/art.10312
Xu D, Song M, Chai C, Wang J, Jin C, Wang X, Cheng M, Yan S (2019) Exosome-encapsulated miR-6089 regulates inflammatory response via targeting TLR4. J Physiol 234(2):1502–1511. https://doi.org/10.1002/jcp.27014
Marton N, Kovács OT, Baricza E, Kittel Á, Győri D, Mócsai A, Meier FMP, Goodyear CS, McInnes IB, Buzás EI, Nagy G (2017) Extracellular vesicles regulate the human osteoclastogenesis: divergent roles in discrete inflammatory arthropathies. and. Mol Life Sci 74(19):3599–3611. https://doi.org/10.1007/s00018-017-2535-8
Gitz E, Pollitt AY, Gitz-Francois JJ, Alshehri O, Mori J, Montague S, Nash GB, Douglas MR, Gardiner EE, Andrews RK, Buckley CD, Harrison P, Watson SP (2014) CLEC-2 expression is maintained on activated platelets and on platelet microparticles. Blood 124(14):2262–2270. https://doi.org/10.1182/blood-2014-05-572818
Greisen SR, Yan Y, Hansen AS, Venø MT, Nyengaard JR, Moestrup SK, Hvid M, Freeman GJ, Kjems J, Deleuran B (2017) Extracellular vesicles transfer the receptor programmed death-1 in rheumatoid arthritis. Front Immunol 8(JUL). https://doi.org/10.3389/fimmu.2017.00851
György B, Szabó TG, Turiák L, Wright M, Herczeg P, Lédeczi Z, Kittel Á, Polgár A, Tóth K, Dérfalvi B, Zelenák G, Böröcz I, Carr B, Nagy G, Vékey K, Gay S, Falus A, Buzás EI (2012) Improved Flow Cytometric Assessment Reveals Distinct Microvesicle (Cell-Derived Microparticle) Signatures in Joint Diseases. PLoS ONE 7(11):e49726. https://doi.org/10.1371/journal.pone.0049726
Fan W, Wang W, Wu J, Ma L, Guo J (2017) Identification of CD4+ T-cell-derived CD161+ CD39+ and CD39+CD73+ microparticles as new biomarkers for rheumatoid arthritis. Biomark Med 11(2):107–116. https://doi.org/10.2217/bmm-2016-0261
Umekita K, Hidaka T, Ueno S, Takajo I, Kai Y, Nagatomo Y, Sawaguchi A, Suganuma T, Okayama A (2009) Leukocytapheresis (LCAP) decreases the level of platelet-derived microparticles (MPs) and increases the level of granulocytes-derived MPs: A possible connection with the effect of LCAP on rheumatoid arthritis. Mod Rheumatol 19(3):265–272. https://doi.org/10.1007/s10165-009-0164-2
Messer L, Alsaleh G, Freyssinet JM, Zobairi F, Leray I, Gottenberg JE, Sibilia J, Toti-Orfanoudakis F, Wachsmann D (2009) Microparticle-induced release of B-lymphocyte regulators by rheumatoid synoviocytes. Res Ther 11(2):R40. https://doi.org/10.1186/ar2648
Jüngel A, Distler O, Schulze-Horsel U, Huber LC, Ha HR, Simmen B, Kalden JR, Pisetsky DS, Gay S, Distler JHW (2007) Microparticles stimulate the synthesis of prostaglandin E(2) via induction of cyclooxygenase 2 and microsomal prostaglandin E synthase 1. Arthritis Rheum 56(11):3564–3574. https://doi.org/10.1002/art.22980
Wang Y, Zheng F, Gao G, Yan S, Zhang L, Wang L, Cai X, Wang X, Xu D, Wang J (2018) MiR-548a-3p regulates inflammatory response via TLR4/NF-κB signaling pathway in rheumatoid arthritis. J Cell Biochem. https://doi.org/10.1002/jcb.26659
Szabó-Taylor KÉ, Tóth EÁ, Balogh AM, Sódar BW, Kádár L, Pálóczi K, Fekete N, Németh A, Osteikoetxea X, Vukman KV, Holub M, Pállinger É, Nagy G, Winyard PG, Buzás EI (2017) Monocyte activation drives preservation of membrane thiols by promoting release of oxidised membrane moieties via extracellular vesicles. Free Radic Biol Med 108:56–65. https://doi.org/10.1016/j.freeradbiomed.2017.03.016
Headland SE, Jones HR, Norling LV, Kim A, Souza PR, Corsiero E, Gil CD, Nerviani A, Dell'accio F, Pitzalis C, Oliani SM, Jan LY, Perretti M (2015) Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci Transl Med 7(315):315ra190. https://doi.org/10.1126/scitranslmed.aac5608
Chen X-M, Zhao Y, Wu X-D, Wang M-J, Yu H, Lu J-J, Hu Y-J, Huang Q-C, Huang R-Y, Lu C-J (2019) Novel findings from determination of common expressed plasma exosomal microRNAs in patients with psoriatic arthritis, psoriasis vulgaris, rheumatoid arthritis, and gouty arthritis. Discov Med 28(151):47–68
Oba R, Isomura M, Igarashi A, Nagata K (2019) Circulating CD3+HLA-DR+extracellular vesicles as a marker for Th1/Tc1-Type immune responses. J Immunol Res 2019:1–13. https://doi.org/10.1155/2019/6720819
Villar-Vesga J, Grajales C, Burbano C, Vanegas-García A, Muñoz-Vahos CH, Vásquez G, Rojas M, Castaño D (2019) Platelet-derived microparticles generated in vitro resemble circulating vesicles of patients with rheumatoid arthritis and activate monocytes. Immunology 336:1–11. https://doi.org/10.1016/j.cellimm.2018.12.002
Reich N, Beyer C, Gelse K, Akhmetshina A, Dees C, Zwerina J, Schett G, Distler O, Distler JHW (2011) Microparticles stimulate angiogenesis by inducing ELR(+) CXC-chemokines in synovial fibroblasts. J Cell Mol Med 15(4):756–762. https://doi.org/10.1111/j.1582-4934.2010.01051.x
Michael BNR, Misra DP, Chengappa KG, Negi VS (2018) Relevance of elevated microparticles in peripheral blood and synovial fluid of patients with rheumatoid arthritis. Indian J Rheumatol 13(4):222–226. https://doi.org/10.4103/injr.injr_101_18
Liao TL, Hsieh SL, Chen YM, Chen HH, Liu HJ, Lee HC, Chen DY (2018) Rituximab May Cause Increased Hepatitis C Virus Viremia in Rheumatoid Arthritis Patients Through Declining Exosomal MicroRNA-155. and. Rheumatology 70(8):1209–1219. https://doi.org/10.1002/art.40495
Rodríguez-Carrio J, Alperi-López M, López P, Ballina-García FJ, Suárez A (2015) Good response to tumour necrosis factor alpha blockade results in an angiogenic T cell recovery in rheumatoid arthritis patients. Rheumatology (United Kingdom) 54(6):1129–1131. https://doi.org/10.1093/rheumatology/kev025
Sellam J, Proulle V, Jüngel A, Ittah M, Miceli Richard C, Gottenberg J-E, Toti F, Benessiano J, Gay S, Freyssinet J-M, Mariette X (2009) Increased levels of circulating microparticles in primary Sjögren's syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther 11(5):R156–R156. https://doi.org/10.1186/ar2833
Berckmans RJ, Nieuwland R, Kraan MC, Schaap MC, Pots D, Smeets TJ, Sturk A, Tak PP (2005) Synovial microparticles from arthritic patients modulate chemokine and cytokine release by synoviocytes. Res Ther 7(3):R536–R544
Tsuno H, Arito M, Suematsu N, Sato T, Hashimoto A, Matsui T, Omoteyama K, Sato M, Okamoto K, Tohma S, Kurokawa MS, Kato T (2018) A proteomic analysis of serum-derived exosomes in rheumatoid arthritis. BMC Rheumatol 2:35–35. https://doi.org/10.1186/s41927-018-0041-8
Viñuela-Berni V, Doníz-Padilla L, Figueroa-Vega N, Portillo-Salazar H, Abud-Mendoza C, Baranda L, González-Amaro R (2015) Proportions of several types of plasma and urine microparticles are increased in patients with rheumatoid arthritis with active disease. Clin Exp Immunol 180(3):442–451. https://doi.org/10.1111/cei.12598
Yoo J, Lee SK, Lim M, Sheen D, Choi E-H, Kim SA (2017) Exosomal amyloid A and lymphatic vessel endothelial hyaluronic acid receptor-1 proteins are associated with disease activity in rheumatoid arthritis. Arthritis Res Ther 19(1):119–119. https://doi.org/10.1186/s13075-017-1334-9
Zhang H-G, Liu C, Su K, Yu S, Zhang L, Zhang S, Wang J, Cao X, Grizzle W, Kimberly RP (2006) A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J Immunol 176(12):7385–7393. https://doi.org/10.4049/jimmunol.176.12.7385
Tofino-Vian M, Guillen MI, Alcaraz MJ (2018) Extracellular vesicles: A new therapeutic strategy for joint conditions. Biochem Pharmacol 153:134–146. https://doi.org/10.1016/j.bcp.2018.02.004
Funding
Open Access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Disclosures
None.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 43.9 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schioppo, T., Ubiali, T., Ingegnoli, F. et al. The role of extracellular vesicles in rheumatoid arthritis: a systematic review. Clin Rheumatol 40, 3481–3497 (2021). https://doi.org/10.1007/s10067-021-05614-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05614-w