Abstract
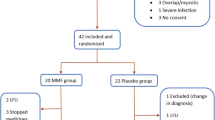
The study aims to analyze the effects of induction treatment with cyclophosphamide (CYC) pulse therapy followed by maintenance treatment with other mild immunosuppressive agents on lung function in scleroderma (SSc) patients. Thirty patients with SSc (mean age 52 years, mean disease duration < 2 years) with forced vital capacity (FVC) ≤ 80% and/or diffusing capacity of carbon monoxide (DLco) ≤ 70% were included. Monthly CYC pulses were given for 6 months (induction treatment), followed by 3-monthly maintenance pulses for the next 18 months, and during the next 5 years patients received other mild immunosupressive therapy brought by the competent rheumatologist. The efficacy was evaluated by comparing FVC% and DLco% after 6, 24, and 84 months from the baseline. All patients completed induction and maintenance treatment with CYC. Three patients were lost to follow-up. The rest of 27 patients, during the next 5 years, received other immunosupressive agents (14 azathioprine, 9 methotrexate, and 4 mycophenolate mofetil). Three patients died in the 4 years of follow-up. By 6, 24, and 84 months, the mean FVC and DLco changes were + 0.47 and + 2.10, + 3.30 and − 2.49, and + 1.53 and − 3.76%, respectively. These changes were not significantly different from the baseline values. CYC does not appear to result in clinically significant improvement of pulmonary function but fulfilled criteria of stable disease. Maintenance treatment with other mild immunosupressive agents preserves the benefits achieved during CYC treatment.

Similar content being viewed by others
References
Steen VD, Conte C, Owens GR, Medsger TA Jr (1994) Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum 37:1283–1289
White B (2003) Interstitial lung disease in scleroderma. Rheum Dis Clin N Am 29:371–390
Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS et al (2006) A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum 54:3962–3970
Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, Arriola E, Silver R, Strange C, Bolster M, Seibold JR, Riley DJ, Hsu VM, Varga J, Schraufnagel DE, Theodore A, Simms R, Wise R, Wigley F, White B, Steen V, Read C, Mayes M, Parsley E, Mubarak K, Connolly MK, Golden J, Olman M, Fessler B, Rothfield N, Metersky M, Scleroderma Lung Study Research Group (2006) Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 354:2655–2666
Cappelli S, Guiducci S, Bellando Randone S, Matucci Cerinic M (2013) Immunosuppression for interstitial lung disease in systemic sclerosis. Eur Respir Rev 22:236–243
American Thoracic Society (2000) Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 16:646–664
Karch FE, Lassagna L (1977) Towards the operational identification of adverse drug reactions. Clin Pharmacol Ther 21:247–254
Kowal-Bielecka O, Landewé R, Avouac J, Chwiesko S, Miniati I, Czirjak L et al (2009) EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR scleroderma trials and research group (EUSTAR). Ann Rheum Dis 68:620–628
Simeón-Aznar CP, Fonollosa-Plá V, Tolosa-Vilella C, Selva-O’ Callaghan A, Solans-Laqué R, Palliza E et al (2008) Intravenous cyclophosphamide pulse therapy in the treatment of systemic sclerosis-related interstitial lung disease: a long term study. Open Respir Med J 2:39–45
Be’rezne’ A, Ranque B, Valeyre D, Brauner M, Allanore Y, Launay D et al (2008) Therapeutic strategy combining intravenous cyclophosphamide followed by oral azathioprine to treat worsening interstitial lung disease associated with systemic sclerosis: a retrospective multicenter open-label study. J Rheumatol 35:1064–1072
Iudici M, Cuomo G, Vettori S, Bocchino M, Sanduzzi Zamparelli A, Cappabianca S, Valentini G (2015) Low-dose pulse cyclophosphamide in interstitial lung disease associated with systemic sclerosis (SSc-ILD): efficacy of maintenance immunosuppression in responders and non-responders. Semin Arthritis Rheum 44:437–444
Ostojic P, Damjanov N (2006) Improvement of lung function in patients with systemic sclerosis after 6 months cyclophosphamide pulse therapy. Clin Rheumatol 25:819–821
Airò P, Danieli E, Rossi M, Frassi M, Cavazzana I, Scarsi M, Grottolo A, Franceschini F, Zambruni A (2007) Intravenous cyclophosphamide for interstitial lung disease associated to systemic sclerosis: results with an 18-month long protocol including a maintenance phase. Clin Exp Rheumatol 25:293–296
Yiannopoulos G, Pastromas V, Antonopoulos I, Katsiberis G, Kalliolias G, Liossis SN, Andonopoulos AP (2007) Combination of intravenous pulses of cyclophosphamide and methylprednizolone in patients with systemic sclerosis and interstitial lung disease. Rheumatol Int 27:357–361
Poormoghim H, Moradi Lakeh M, Mohammadipour M, Sodagari F, Toofaninjed N (2012) Cyclophosphamide for scleroderma lung disease: a systematic review and meta-analysis. Rheumatol Int 32:2431–2444
Tashkin DP, Elashoff R, Clements PJ, Roth MD, Furst DE, Silver RM et al (2007) Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med 76:1026–1034
Broad K, Pope JE (2010) The efficacy of treatment for systemic sclerosis interstitial lung disease: results from a meta-analysis. Med Sci Monit 16:RA187–RA190
Nannini C, West CP, Erwin PJ, Matteson EL (2008) Effects of cyclophosphamide on pulmonary function in patients with scleroderma and interstitial lung disease: a systematic review and meta-analysis of randomized controlled trials and observational prospective cohort studies. Arthritis Res Ther 10:R124
Hudson M, Steele R, Canadian Scleroderma Research Group, Baron M (2012) Immunosuppression for interstitial lung disease in systemic sclerosis - novel insights and opportunities for translational research. J Cell Commun Signal 6:187–190
Poormoghim H, Rezaei N, Sheidaie Z, Almasi AR, Moradi-Lakeh M, Almasi S, Andalib E (2014) Systemic sclerosis: comparison of efficacy of oral cyclophosphamide and azathioprine on skin score and pulmonary involvement-a retrospective study. Rheumatol Int 34:1691–1699
Nadashkevich O, Davis P, Fritzler M, Kovalenko W (2006) A randomized unblinded trial of cyclophosphamide versus azathioprine in the treatment of systemic sclerosis. Clin Rheumatol 25:205–212
Arakawa H, Yamasaki M, Kurihara Y, Yamada H, Nakajima Y (2003) Methotrexate induced pulmonary injury: serial CT findings. J Thorac Imaging 18:231–236
Panopoulos ST, Bournia VK, Trakada G, Giavri I, Kostopoulos C, Sfikakis PP (2013) Mycophenolate versus cyclophosphamide for progressive interstitial lung disease associated with systemic sclerosis: a 2-year case control study. Lung 191:483–489
Adler S, Huscher D, Siegert E, Allanore Y, Czirják L, DelGaldo F et al (2018) Systemic sclerosis associated interstitial lung disease - individualized immunosuppressive therapy and course of lung function: results of the EUSTAR group. Arthritis Res Ther 20:17
Bruni C, Guiducci S, Bellando-Randone S, Lepri G, Braschi F, Fiori G, Bartoli F, Peruzzi F, Blagojevic J, Matucci-Cerinic M (2015) Digital ulcers as a sentinel sign for early internal organ involvement in very early systemic sclerosis. Rheumatology (Oxford) 54:72–76
Valentini G, Marcoccia A, Cuomo G, Vettori S, Iudici M, Bondanini F, Santoriello C, Ciani A, Cozzolino D, de Matteis GM, Cappabianca S, Vitelli F, Spanò A (2013) Early systemic sclerosis: marker autoantibodies and videocapillaroscopy patterns are each associated with distinct clinical, functional and cellular activation markers. Arthritis Res Ther 15:R63
Sakkas LI, Simopoulou T, Katsiari C, Bogdanos D, Chikanza IC (2015) Early systemic sclerosis-opportunities for treatment. Clin Rheumatol 34:1327–1331
Ciurzyński M, Bienias P, Lichodziejewska B, Kurnicka K, Szewczyk A, Glińska-Wielochowska M, Kurzyna M, Błaszczyk M, Liszewska-Pfejfer D, Pruszczyk P (2008) Non-invasive diagnostic and functional evaluation of cardiac involvement in patients with systemic sclerosis. Clin Rheumatol 27:991–997
Allanore Y, Borderie D, Avouac J, Zerkak D, Meune C, Hachulla E, Mouthon L, Guillevin L, Meyer O, Ekindjian OG, Weber S, Kahan A (2008) High N-terminal pro-brain natriuretic peptide levels and low diffusing capacity for carbon monoxide as independent predictors of the occurrence of precapillary pulmonary arterial hypertension in patients with systemic sclerosis. Arthritis Rheum 58:284–291
Choi HJ, Shin YK, Lee HJ, Kee JY, Shin DW, Lee EY, Lee YJ, Lee EB, Song YW (2008) The clinical significance of serum N-terminal pro-brain natriuretic peptide in systemic sclerosis patients. Clin Rheumatol 27:437–442
Dimitroulas T, Giannakoulas G, Papadopoulou K, Sfetsios T, Karvounis H, Dimitroula H, Parcharidou D, Koliakos G, Garyfallos A, Styliadis I, Settas L (2010) Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 29:957–964
Maurer B, Graf N, Michel BA, Müller-Ladner U, Czirják L, Denton CP, Tyndall A, Metzig C, Lanius V, Khanna D, Distler O, EUSTAR co-authors (2015) Prediction of worsening of skin fibrosis in patients with diffuse cutaneous systemic sclerosis using the EUSTAR database. Ann Rheum Dis 74:1124–1131
Naraghi K, van Laar JM (2013) Update on stem cell transplantation for systemic sclerosis: recent trial results. Curr Rheumatol Rep 15:326–331
Del Papa N, Onida F, Zaccara E, Saporiti G, Maglione W, Tagliaferri E et al (2017) Autologous hematopoietic stem cell transplantation has better outcomes than conventional therapies in patients with rapidly progressive systemic sclerosis. Bone Marrow Transplant 52:53–58
van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, Schuerwegh AJ, Marijt EW, Vonk MC, Schattenberg AV, Matucci-Cerinic M, Voskuyl AE, van de Loosdrecht A, Daikeler T, Kötter I, Schmalzing M, Martin T, Lioure B, Weiner SM, Kreuter A, Deligny C, Durand JM, Emery P, Machold KP, Sarrot-Reynauld F, Warnatz K, Adoue DF, Constans J, Tony HP, del Papa N, Fassas A, Himsel A, Launay D, Lo Monaco A, Philippe P, Quéré I, Rich É, Westhovens R, Griffiths B, Saccardi R, van den Hoogen F, Fibbe WE, Socié G, Gratwohl A, Tyndall A, EBMT/EULAR Scleroderma Study Group (2014) Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA 311:2490–2498
Helbig G, Widuchowska M, Koclęga A, Kopińska A, Kopeć-Mędrek M, Gaweł WB, Spałek A, Żak J, Grygoruk-Wiśniowska I, Liwoch R, Kucharz E, Markiewicz M (2018) Safety profile of autologous hematopoietic stem cell mobilization and transplantation in patients with systemic sclerosis. Clin Rheumatol 37:1709–1714
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Pavlov-Dolijanovic, S., Vujasinovic Stupar, N., Zugic, V. et al. Long-term effects of immunosuppressive therapy on lung function in scleroderma patients. Clin Rheumatol 37, 3043–3050 (2018). https://doi.org/10.1007/s10067-018-4266-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4266-0