Abstract
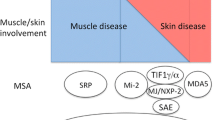
Inflammatory myopathies are a clinically diverse group of diseases, in which the detection of particular autoantibodies may facilitate diagnosis, treatment, and prognosis. The aim of this report is to summarize our experience with specific autoantibody testing in patients with inflammatory myopathies. Data were collected over the last decade in the Autoimmune Center of the Sheba Medical Center, a tertiary referral hospital. Data regarding patients’ positive for autoantibodies against Jo-1, PL-7, PL-12, SRP, Mi-2, Ku, and PM-Scl antigens were retrospectively collected. Patient demographics, clinical characteristics, and mortality were recorded. Descriptive statistics (mean, standard deviation, frequency, and percentage) were calculated. A total of 507 patients were surveyed for sclero-poly-synthetase antibodies, as part of the diagnostic workup of myositis/myalgia or interstitial lung disease. Forty-three patients were found positive for one or more of the abovementioned antibodies, and 23 of them (53.49%) had interstitial lung disease (ILD). Four patients were positive for anti-PL-7, three of them had ILD and Raynaud’s phenomenon. Five patients were positive for anti-Ku, and four of them had both arthritis and Raynaud’s phenomenon. Nine patients were positive for anti-Mi-2, and six of them were given diagnosed with dermatomyositis. Ten patients were positive for anti-SRP, and six of them had cancers of various types. Our results reiterate the previously recognized associations between anti-Mi-2 and dermatomyositis, anti-Ku and Raynaud’s phenomenon, and between anti-PL-7 and ILD. In addition, our data support an association between anti-SRP autoantibody positivity and malignancy, which calls for further investigation.

Similar content being viewed by others
References
Meyer A, Meyer N, Schaeffer M, Gottenberg JE, Geny B, Sibilia J (2015) Incidence and prevalence of inflammatory myopathies: a systematic review. Rheumatology 54:50–63. https://doi.org/10.1093/rheumatology/keu289
Dobloug C, Garen T, Bitter H et al (2015) Prevalence and clinical characteristics of adult polymyositis and dermatomyositis; data from a large and unselected Norwegian cohort. Ann Rheum Dis 74:1551–1556. https://doi.org/10.1136/annrheumdis-2013-205127
Dobloug GC, Antal EA, Sveberg L, Garen T, Bitter H, Stjärne J, Grøvle L, Gran JT, Molberg Ø (2015) High prevalence of inclusion body myositis in Norway; a population-based clinical epidemiology study. Eur J Neurol 22:672–e41. https://doi.org/10.1111/ene.12627
Ghirardello A, Bassi N, Palma L, Borella E, Domeneghetti M, Punzi L, Doria A (2013) Autoantibodies in polymyositis and dermatomyositis. Curr Rheumatol Rep 15:335. https://doi.org/10.1007/s11926-013-0335-1
Brouwer R, Vree Egberts WTM, Hengstman GJD, Raijmakers R, van Engelen BGM, Peter Seelig H, Renz M, Mierau R, Genth E, Pruijn GJM, van Venrooij WJ (2002) Autoantibodies directed to novel components of the PM/Scl complex, the human exosome. Arthritis Res 4:134–138. https://doi.org/10.1186/ar389
Allmang C, Petfalski E, Podtelejnikov A, Mann M, Tollervey D, Mitchell P (1999) The yeast exosome and human PM-Scl are related complexes of 3′→5′ exonucleases. Genes Dev 13:2148–2158. https://doi.org/10.1101/gad.13.16.2148
De La TIG (2015) Clinical usefulness of autoantibodies in idiopathic inflammatory myositis. Front Immunol 6:331. https://doi.org/10.3389/fimmu.2015.00331
Betteridge ZE, Gunawardena H, McHugh NJ (2011) Novel autoantibodies and clinical phenotypes in adult and juvenile myositis. Arthritis Res Ther 13:209. https://doi.org/10.1186/ar3275
Zhang Y, LeRoy G, Seelig HP, Lane WS, Reinberg D (1998) The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell 95:279–289. https://doi.org/10.1016/S0092-8674(00)81758-4
Mahler M, Miller FW, Fritzler MJ (2014) Idiopathic inflammatory myopathies and the anti-synthetase syndrome: a comprehensive review. Autoimmun Rev 13:367–371. https://doi.org/10.1016/j.autrev.2014.01.022
Targoff IN, Johnson AE, Miller FW (1990) Antibody to signal recognition particle in polymyositis. Arthritis Rheum 33:1361–1370. https://doi.org/10.1002/art.1780330908
Mierau R, Dick T, Bartz-Bazzanella P, Keller E, Albert ED, Genth E (1996) Strong association of dermatomyositis-specific Mi-2 autoantibodies with a tryptophan at position 9 of the HLA-DR beta chain. Arthritis Rheum 39:868–876. https://doi.org/10.1002/art.1780390521
Hervier B, Wallaert B, Hachulla E, Adoue D, Lauque D, Audrain M, Camara B, Fournie B, Couret B, Hatron PY, Dubucquoi S, Hamidou M (2010) Clinical manifestations of anti-synthetase syndrome positive for anti-alanyl-tRNA synthetase (anti-PL12) antibodies: a retrospective study of 17 cases. Rheumatology (Oxford) 49:972–976. https://doi.org/10.1093/rheumatology/kep455
Marguerie C, Bunn CC, Beynon HLC, et al (1990) Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes. 1019–1038
Sauty A, Rochat T, Schoch OD, Hamacher J, Kurt AM, Dayer JM, Nicod LP (1997) Pulmonary fibrosis with predominant CD8 lymphocytic alveolitis and anti-jo-1 antibodies. Eur Respir J 10:2907–2912. https://doi.org/10.1183/09031936.97.10122907
Koenig M, Fritzler MJ, Targoff IN, Troyanov Y, Senécal JL (2007) Heterogeneity of autoantibodies in 100 patients with autoimmune myositis: insights into clinical features and outcomes. Arthritis Res Ther 9:R78. https://doi.org/10.1186/ar2276
Yoshifuji H, Fujii T, Kobayashi S, Imura Y, Fujita Y, Kawabata D, Usui T, Tanaka M, Nagai S, Umehara H, Mimori T (2006) Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity 39:233–241. https://doi.org/10.1080/08916930600622884
Stone KB, Oddis CV, Fertig N, Katsumata Y, Lucas M, Vogt M, Domsic R, Ascherman DP (2007) Anti-Jo-1 antibody levels correlate with disease activity in idiopathic inflammatory myopathy. Arthritis Rheum 56:3125–3131. https://doi.org/10.1002/art.22865
Targoff IN, Reichlin M (1985) The association between Mi-2 antibodies and dermatomyositis. Arthritis Rheum 28:796–803. https://doi.org/10.1002/art.1780280711
Ghirardello A, Zampieri S, Iaccarino L, Tarricone E, Bendo* R, Gambari PF, Doria A (2005) Anti-Mi-2 antibodies. Autoimmunity 38:79–83. https://doi.org/10.1080/08916930400022681
Hervier B, Uzunhan Y, Hachulla E, Benveniste O, Nunes H, Delaval P, Musset L, Dubucquoi S, Wallaert B, Hamidou M (2011) Antisynthetase syndrome positive for anti-threonyl-tRNA synthetase (anti-PL7) antibodies. Eur Respir J 37:714–717. https://doi.org/10.1183/09031936.00104310
Marie I, Josse S, Decaux O, Diot E, Landron C, Roblot P, Jouneau S, Hatron PY, Hachulla E, Vittecoq O, Menard JF, Jouen F, Dominique S (2013) Clinical manifestations and outcome of anti-PL7 positive patients with antisynthetase syndrome. Eur J Intern Med 24:474–479. https://doi.org/10.1016/j.ejim.2013.01.002
Labirua-Iturburu A, Selva-O’Callaghan A, Vincze M, Dankó K, Vencovsky J, Fisher B, Charles P, Dastmalchi M, Lundberg IE (2012) Anti-PL-7 (anti-threonyl-tRNA synthetase) antisynthetase syndrome: clinical manifestations in a series of patients from a European multicenter study (EUMYONET) and review of the literature. Med 91:206–211. https://doi.org/10.1097/MD.0b013e318260977c
Yamasaki Y, Yamada H, Nozaki T, Akaogi J, Nichols C, Lyons R, Chin Loy A, Chan EKL, Reeves WH, Satoh M (2006) Unusually high frequency of autoantibodies to PL-7 associated with milder muscle disease in Japanese patients with polymyositis/dermatomyositis. Arthritis Rheum 54:2004–2009. https://doi.org/10.1002/art.21883
Friedman a W, Targoff IN, Arnett FC (1996) Interstitial lung disease with autoantibodies against aminoacyl-tRNA synthetases in the absence of clinically apparent myositis. Semin Arthritis Rheum 26:459–467. https://doi.org/10.1016/S0049-0172(96)80026-6
Franceschini F, Cavazzana I, Generali D, Quinzanini M, Viardi L, Ghirardello A, Doria A, Cattaneo R (2002) Anti-Ku antibodies in connective tissue diseases: clinical and serological evaluation of 14 patients. J Rheumatol 29:1393–1397
Cavazzana I, Ceribelli A, Quinzanini M, Scarsi M, Airò P, Cattaneo R, Franceschini F (2008) Prevalence and clinical associations of anti-Ku antibodies in systemic autoimmune diseases. Lupus 17:727–732. https://doi.org/10.1177/0961203308089442
Mastaglia FL, Phillips BA (2002) Idiopathic inflammatory myopathies: epidemiology, classification, and diagnostic criteria. Rheum Dis Clin N Am 28:723–741. https://doi.org/10.1016/S0889-857X(02)00021-2
Basnayake SK, Blumbergs P, Tan JA et al (2015) Inflammatory myopathy with anti-SRP antibodies: case series of a South Australian cohort. Clin Rheumatol 34:603–608. https://doi.org/10.1007/s10067-014-2512-7
Hengstman GJ, ter Laak HJ, Vree Egberts WT et al (2006) Anti-signal recognition particle autoantibodies: marker of a necrotising myopathy. Ann Rheum Dis 65:1635–1638. https://doi.org/10.1136/ard.2006.052191
Suzuki S, Nishikawa A, Kuwana M, Nishimura H, Watanabe Y, Nakahara J, Hayashi YK, Suzuki N, Nishino I (2015) Inflammatory myopathy with anti-signal recognition particle antibodies: case series of 100 patients. Orphanet J Rare Dis 10:61. https://doi.org/10.1186/s13023-015-0277-y
Rodríguez-Muguruza S, Lozano-Ramos I, Canti JC et al (2017) Anti-SRP auto-antibodies are not specific for myositis: report of 8 cases. Joint Bone Spine 84:103–105. https://doi.org/10.1016/j.jbspin.2015.12.005
Martel C, Vignaud G, Liozon E, Magy L, Gallouedec G, Ly K, Bezanahary H, Cypierre A, Lapébie FX, Palat S, Gondran G, Jauberteau MO, Fauchais AL (2016) Contribution of dot-blot assay to the diagnosis and management of myositis: a three-year practice at a university hospital centre. Clin Exp Rheumatol 34:918–924
Chen Z, Cao M, Plana MN, Liang J, Cai H, Kuwana M, Sun L (2013) Utility of anti-melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res 65:1316–1324. https://doi.org/10.1002/acr.21985
Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C et al (2010) Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med 123:558–562. https://doi.org/10.1016/j.amjmed.2009.11.012
Acknowledgments
The authors thank Jacqueline Wyatt for her contribution in editing and proofreading of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical considerations are detailed in methods.
Disclosures
None.
Rights and permissions
About this article
Cite this article
Gofrit, S.G., Yonath, H., Lidar, M. et al. The clinical phenotype of patients positive for antibodies to myositis and myositis-related disorders. Clin Rheumatol 37, 1257–1263 (2018). https://doi.org/10.1007/s10067-018-4032-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4032-3