Abstract
Hepatitis C-associated osteosclerosis (HCAO) is an impressive example of acquired diffuse osteosclerosis in adults, recently described in ten patients infected with hepatitis C virus (HCV). Its hallmark is a painful and generalized increase of bone mass. Bone biopsies show enhanced accretion rate, usually without histological abnormalities. The HCAO pathogenesis is hitherto unknown. HCV might induce a slow bone cell infection and the production of bone growth factors, such as insulin-like growth factors. Recently, receptor activator of nuclear factor-κB (RANK), its ligand (RANKL), and soluble decoy receptor osteoprotegerin (OPG) have been identified as a pivotal cytokine system in the bone remodeling control. We describe the 11th case of HCAO. Notably, the patient’s bone biopsy showed the presence of a high number of OPG-positive osteoblasts, a slight increase of RANKL-positive stromal cells, and a dramatic reduction of the osteoclasts. Moreover, OPG serum levels were increased. These findings reported here for the first time are consistent with a pathogenetic role of the OPG/RANKL system imbalance in HCAO.
Similar content being viewed by others
Introduction
Hepatitis C-associated osteosclerosis (HCAO) is an acquired, painful skeletal disorder characterized by a marked increase of bone mass, recently described in ten adults infected with the hepatitis C virus (HCV) [1–9]. The pathogenesis of this rare syndrome is so far unknown. It has been suggested that HCV and/or another unidentified parenterally transmitted agent may cause a slow infection of bone cells or their precursors in predisposed subjects [7]. In fact, only a very small percentage of all HCV-infected patients develops osteosclerosis, perhaps mediated by the production of bone growth factors, such as insulin-like growth factors (IGFs) [10].
In recent years, receptor activator of nuclear factor-κB (RANK), its ligand (RANKL), and osteoprotegerin (OPG) have been identified as a pivotal cytokine system in the control of bone remodeling [11]. RANKL, a member of the tumor necrosis factor ligand superfamily expressed by bone marrow stromal/osteoblastic cells and activated T lymphocytes, plays an important role in the formation, activation, and survival of osteoclasts, by binding to its specific receptor RANK, located on osteoclast progenitors [11]. The effects of the RANK/RANKL interaction are counteracted by OPG, secreted as a decoy receptor by osteoblastic cells [11]. The critical role of the RANK/RANKL/OPG system in regulating bone homeostasis is demonstrated by the findings of extreme skeletal phenotypes (osteoporosis versus osteopetrosis) in mice with altered expression of these cytokines [11]. Notably, in patients affected by multiple myeloma (MM) with osteolytic lesions, an imbalance of OPG/RANKL ratio, in favor of RANKL, has been recently demonstrated in bone environment [12].
In this report, describing the 11th hitherto diagnosed case of HCAO, we examine the hypothesis that an alteration of the OPG/RANKL system might contribute to the increase in bone mass.
Materials and methods
Osteoprotegerin serum levels were measured by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, Minn., USA) and bone mineral density (BMD) by dual-energy X-ray absorptiometry (DEXA) (QDR4500A, Hologic, Waltham, Mass., USA).
The patient’s bone specimen, obtained by transiliac biopsy, was formalin fixed, decalcified by ethylenediaminetetraacetate (EDTA) and embedded in paraffin. Sections were stained with hematoxylin and eosin. Serial sections (3-μm thick) were processed for immunohistochemical staining with antihuman (h) OPG monoclonal antibody (MAb) (clone: 69,127.11, R&D Systems, Minneapolis, Minn., USA; working dilution 1:100) or anti-hRANKL MAb (clone: 70,525.11, R&D Systems, Minneapolis, Minn., USA; working dilution 1:25), using the immunoperoxidase technique (DAKO LSAB2 System, HRP, Carpinteria, Calif., USA). Briefly, endogenous peroxidase activity was blocked with 3% H2O2 for 8 min. Slides were incubated with anti-OPG and anti-RANKL MAbs for 60 and 30 min, respectively, followed by biotinylated anti-mouse IgG and streptavidin-horseradish peroxidase for 10 min. Finally, the peroxidase reaction was developed with 3,3′-diaminobenzidine and nuclei were counterstained with hematoxylin. All incubations were performed at room temperature. As negative control, primary antibodies were omitted or replaced with an isotype control-matched MAb with irrelevant specificity. The control group was represented by bone biopsies obtained from three patients with HCV-related chronic hepatitis and without bone alterations, and from five patients with osteolytic MM. The latter group was included since we have previously demonstrated the role of the OPG/RANKL system in the pathologic bone remodeling of osteolytic MM [12].
Case report
A 74-year-old retired policeman came under our observation in December 2001, because of the worsening and extension of deep pain persisting for 10 years in his knees and legs, in the absence of either joint swelling or limitation of motion. Physical therapies as well as several nonsteroidal anti-inflammatory drugs did not result in any clinical improvement. His past medical history was negative for blood transfusions, drug abuse, or tattoos. In 1991, he underwent cholecystectomy for gallbladder stones. At that time, the HCV antibody test was negative, whereas the result was positive 2 years later. His two sons and 69-year-old sister were all in good health, without evidence of skeletal abnormalities.
On physical examination, the patient presented as a tall man (height 180 cm, weight 86 kg) without skeletal deformities. The most relevant clinical finding was a marked, diffuse tenderness in his knees and legs, even to a light palpation of the long bones.
Laboratory investigations showed a mild thrombocytopenia (125,000/mm3), elevated levels of alanine [117 U/l; normal value (NV) <36] and aspartate (76 U/l; NV <36) aminotransferases, as well as alkaline phosphatase (ALP) (1095 U/l; NV <270), due to a 6.8-fold increase of its bone isoenzyme. Serum osteocalcin (24.2 ng/dl; NV <13.70) and OPG values were increased (46 ng/ml; NV, mean±SD: 26.6±4.8) [12]. Age-matched urinary pyridinium cross-link, serum intact parathyroid hormone (iPTH), calcium, phosphorus, 25-hydroxyvitamin D, and plasma fluoride levels were normal. Anti-hepatitis C virus antibodies were detected by second generation enzyme-linked immunoassay and confirmed by recombinant immunoblotting assay. The presence of HCV RNA was revealed by the reverse transcriptase polymerase chain reaction. Autoantibodies and tumor markers were negative.
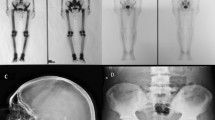
Abdominal and pelvic echography showed marked fibrosis, diffuse fatty liver disease, and prostatic hyperplasia, respectively. Chest X-rays demonstrated a diffuse osteosclerosis of the ribs, in the absence of pleural or pulmonary abnormalities. Skeletal radiographs revealed a diffuse osteosclerosis, mainly involving the diaphyseal cortices (Fig. 1) and sparing the facial bones and skull. These findings were not observed in previous radiograms, performed in 1990 and in 1998. Bone scintigraphy using 99m technetium methylene diphosphonate (99m Tc-MDP) showed an enhanced radionuclide uptake of the lower limb long bones with a double-line pattern. Interestingly, this finding was not seen in a previous bone scan carried out in 1990.
When compared to both the young adult and age-matched sex-specific reference range, the patient’s BMD results were markedly elevated in all regions (L2–L4: T-score +3.75, Z-score +4.74; total hip: T-score +5.46, Z-score +6.20; ultradistal radius: T-score +12.68, Z-score +14.07) as well as in the whole body (T-score +7.09, Z-score +8.10). Moreover, in November 2002, a further increase in BMD values was found with respect to the previous DEXA examination (L2–L4: +4.4%, total hip: +1.9%, ultradistal radius: +6.6%, whole body +3.4%).
The bone biopsy showed an increased number and thickness of bone trabeculae with a parallel reduction of bone marrow space (Fig. 2a). An increase of both activated and quiescent osteoblasts was also found, in the absence of other significant pathological features. The osteoblast density was 7.5/mm2 in HCAO, 3.3±0.6/mm2 in HCV controls, and 4.3±2/mm2 in MM biopsies. Immunostaining showed that the percentage of OPG-labeled osteoblasts (Fig. 2b) in the HCAO specimen was 13.3%, resulting in a twofold and 3.8-fold increase compared to HCV-infected (6.6±3.5% mean±SD) and to osteolytic MM (3.5±2.8% mean±SD) bone marrow samples, respectively.
Bone biopsy of the HCAO patient. a Increased bone density with relative reduction in hematopoietic space is evident (H&E, original magnification ×40). b The intense OPG labeling of osteoblasts is outlined by the magnified detail (immunoperoxidase, original magnification ×200). c Several RANKL-positive cells are present in the bone marrow stroma (immunoperoxidase, original magnification ×400)
Moreover, a marked reduction of the osteoclast population was observed in the HCAO bone. In fact, the osteoclast numerical density was markedly decreased in our patient specimen (2.93/100 mm2) compared to both HCV-positive controls (7±0.5/100 mm2) and osteolytic MM (59±4.4/100 mm2) samples. Finally, the number of RANKL-positive stromal cells (Fig. 2c) in the HCAO specimen (58.6/100 mm2) appeared to be increased when compared to the HCV-infected patient (21.3±9/100 mm2) samples and markedly reduced with respect to those of osteolytic MM patients (110±5.2/100 mm2). Thus, a marked increase in both number and OPG expression of osteoblasts characterized the HCAO bone. Interestingly, the proportion of OPG/RANKL expressing cells may explain the excessive bone apposition of HCAO as opposed to the severely impaired bone remodeling of MM.
Discussion
The patient we describe showed all of the hallmarks of HCAO, including long-lasting appendicular skeletal pain, abnormally high bone density, increased 99m Tc-MDP uptake on bone scintiscan, biochemical parameters consistent with enhanced bone apposition, and HCV infection [1–9]. The patient’s phenotypic features and his familial history, negative for genetic skeletal disorders, excluded both congenital osteopetrosis and pyknodysostosis. The absence of occupational exposure to fluoridated compounds and the normal fluoride plasma levels ruled out the diagnosis of fluorosis. Thus, he represents a new case of HCAO, to our knowledge the 11th hitherto described, being the only one reported from Europe and the oldest one [1–9].
The skeletal pain in HCAO, usually affecting the legs, seems likely related to the periosteal stretching, induced by the new bone apposition [2]. As clearly confirmed in our patient, in HCAO, the facial bones and skull are generally spared, while bone X-ray examination shows osteosclerosis in the appendicular and axial skeleton, with diffuse cortical thickening and usually normal trabecular pattern. Bone mineral density may increase to more than twice the mean values predicted for age and sex [4]. In our case, it should be pointed out that even higher values of BMD were measured. In HCAO, bone biopsy reveals an increased bone formation, usually without significant histopathologic abnormalities [8]. The HCAO skeletal features seem to result from a rapid apposition over a relatively short time, rather than from a slow and long-lasting excessive bone accretion [8]. In our patient, a long period (about 10 years) elapsed between the onset of the clinical symptoms and the radiological expression of the disease. In fact, the skeletal radiographs (performed in 1990 at the beginning of bone pain, and later in 1998) did not detect any osteosclerotic change. Moreover, 1 year after the diagnosis, we documented a further increase in BMD. This finding highlights that, in our patient, the dramatic bone apposition occurred recently and very quickly.
Hepatitis C-associated osteosclerosis is an impressive example of acquired diffuse osteosclerosis in adult life [4]. The pathogenesis of this affection is so far unknown. As suggested for Paget’s disease of bone [13], HCAO might reflect a slow viral infection involving also bone cells in predisposed patients [8, 14]. Individual susceptibility factors likely play an important role, since only a very small percentage of all chronic HCV carriers develops osteosclerosis. HCV may induce the liver and the bone itself to produce bone anabolic factors, such as IGFs [10]. Indeed, HCAO is associated with elevated serum levels of IGF-IIE, an IGF-II precursor [10], and IGF binding protein-2 (IGFBP-2) [10, 14], without significant alterations of IGF-I and II levels or their serum-binding profiles [10]. IGF-IIE and IGFBP-2 circulate together in a bioavailable, approximately 50-kDa complex [10]. On the basis of these findings and in vitro studies, demonstrating high avidity of the IGFII/IGFBP-2 complex for the human osteoblast extracellular matrix, it has been suggested that IGFBP-2 was targeting IGF-IIE to the skeleton in these patients, with consequent stimulation of bone formation [10]. Furthermore, in some cases of HCAO, increased serum values of PTH have been found [8, 14], which may either stimulate or act synergistically with other factors to enhance osteoblast activity. Similarly, PTH may promote the secretion of IGF-I and IGF-II from osteoblasts [15]. However, this mechanism does not seem to have been triggered in our case, since normal iPTH serum levels have been found. Finally, HCV might interfere with the complex cytokine network that controls the bone remodeling [11]. The biologic importance of this system is highlighted by the presence of severe osteoporosis in OPG knockout mice and, on the other hand, the development of osteopetrosis in transgenic mice overexpressing OPG or in RANKL knockout mice [11]. In addition, OPG treatment of normal rats induces a 1.5-fold increase in BMD and 1.8-fold increase in bone volume, in the absence of histopathologic abnormalities [11]. This phenomenon closely mimics the clinical findings of HCAO.
In the case of HCAO reported here, the bone tissue was characterized by a high number of OPG-positive osteoblasts, a moderate increase of RANKL-positive stromal cells, and a severe reduction of osteoclast density. Moreover, OPG serum levels were elevated. These findings, to our knowledge reported for the first time, are consistent with a pathogenetic role of the OPG/RANKL system imbalance in HCAO. Moreover, HCV-infected controls, in the absence of bone alterations, showed a similar ratio between OPG-positive osteoblasts and the RANKL-positive stromal cells with respect to the HCAO patient, suggesting that even a reduced RANKL sensitivity by the osteoclast progenitors may occur in this condition and further contribute to the blunted osteoclastogenesis characteristic of HCAO.
Despite its rarity, HCAO is an amazing example of an acquired bone disorder, and better knowledge of this condition may have potential implications for the treatment of bone disorders. Notwithstanding the limitations of a single case report, the net biological effect of the alterations we describe for the first time in this patient may open new perspectives in the study of such an interesting disease.
Take home message
OPG/RANKL system imbalance may play a role in the pathogenesis of HCAO.
References
Beyer HS, Parfitt AM, Shih MS, Anderson Q, Heath H (1990) Idiopathic acquired diffuse osteosclerosis in a young woman. J Bone Miner Res 5:1257–1263
Villareal DT, Murphy WA, Teitelbaum SL, Arens MQ, Whyte MP (1992) Painful diffuse osteosclerosis after intravenous drug abuse. Am J Med 93:371–381
Whyte MP, Murphy WA, Villareal DT, Teitelbaum SL, Arens MQ (1993) Diffuse osteosclerosis after intravenous drug abuse. Am J Med 95:661–662
Whyte MP, Teitelbaum SL, Reinus WR (1996) Doubling skeletal mass during adult life: the syndrome of diffuse osteosclerosis after intravenous drug abuse. J Bone Miner Res 11:554–558
Diamond T, Depczynsky B (1996) Acquired osteosclerosis associated with intravenous drug use and hepatitis C infection. Bone 19:679–683
Whyte MP, Reasner CA II (1997) Hepatitis C-associated osteosclerosis after blood transfusion. Am J Med 102:219–220
Hassoun AAK, Nippoldt TB, Tiegs RD, Khosla S (1997) Hepatitis C-associated osteosclerosis: an unusual syndrome of acquired osteosclerosis in adults. Am J Med 103:70–73
Shaker JL, Reinus WR, Whyte MP (1998) Hepatitis C-associated osteosclerosis: late onset after blood transfusion in an elderly woman. J Clin Endocrinol Metab 83:93–98
Wakitani S, Hattori T, Nakaya H, Chae YM, Murata N, Tanigami A (2003) Clinical images: hepatitis C-associated osteosclerosis. Arthritis Rheum 48:268
Khosla S, Hassoun AAK, Baker BK, Liu F, Zein NN, Whyte MP, et al (1998) Insulin-like growth factor system abnormalities in hepatitis C-associated osteosclerosis. Potential insights into increasing bone mass in adults. J Clin Invest 101:2165–2173
Hofbauer LC, Heufelder AE (2001) Role of receptor activator of nuclear factor-κB ligand and osteoprotegerin in bone cell biology. J Mol Med 79:243–253
Giuliani N, Bataille R, Mancini C, Lazaretti M, Barillé S (2001) Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood 98:3527–3533
Singer FR (1996) Paget’s disease of bone: possible viral basis. Trends Endocrinol Metab 7:258–261
Shaker JL, Moore BP, Whyte MP (1999) Hyperparathyroidism and increased serum IGF-binding protein-2 levels in hepatitis C-associated osteosclerosis. J Clin Endocrinol Metab 84:384–385
Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R (1993) Anabolic actions of parathyroid hormone on bone. Endocr Rev 14:690–709
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manganelli, P., Giuliani, N., Fietta, P. et al. OPG/RANKL system imbalance in a case of hepatitis C-associated osteosclerosis: the pathogenetic key?. Clin Rheumatol 24, 296–300 (2005). https://doi.org/10.1007/s10067-004-1031-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-004-1031-3