Abstract
To assess and meta-analyse the pooled dropout rate from the randomised control trilas that use virtual reality for balance or gait rehabilitation in people with multiple sclerosis. A systematic review of randomised control trials with meta-analysis and meta-regressions was performed. A search was conducted in PubMed, Scopus, Web of Science, the Physiotherapy Evidence Database, the Cochrane Database, CINHAL, LILACS, ScienceDirect, and ProQuest. It was last updated in July 2022. After the selection of studies, a quality appraisal was carried out using the PEDro Scale and the Revised Cochrane risk-of-bias tool for randomised trials. A descriptive analysis of main characteristics and dropout information was performed. An overall proportion meta-analysis calculated the pooled dropout rate. Odds ratio meta-analysis compared the dropout likelihood between interventions. The meta-regression evaluated the influence of moderators related to dropout. Sixteen studies with 656 participants were included. The overall pooled dropout rate was 6.6% and 5.7% for virtual reality and 9.7% in control groups. The odds ratio (0.89, p = 0.46) indicated no differences in the probability of dropouts between the interventions. The number, duration, frequency, and weeks of sessions, intervention, sex, multiple sclerosis phenotype, Expanded Disability Status Scale score, and PEDro score were not moderators (p > 0.05). Adverse events were not reported and could not be analysed as moderators. Dropouts across the virtual reality and control comparators were similar without significant differences. Nonetheless, there is a slight trend that could favour virtual reality. Standardisation in reporting dropouts and adverse events is recommended for future trials.
PROSPERO database, registration number ID
CRD42021284989.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Different types of virtual reality technology (e.g. non-immersive, semi-immersive, or fully immersive) have emerged as an useful tool in neurorehabilitation with promising results for physical and cognitive rehabilitation (Voinescu et al. 2021). In this way, virtual reality-based interventions have been enhanced as a technological solution for telerehabilitation at the time of the COVID-19 pandemic (Matamala-Gomez et al. 2021). Furthermore, the previous literature has proposed that virtual reality strategies present higher adherence in patients with neurological disorders (Asadzadeh et al. 2021; Dalmazane et al. 2021). Multitask training, patient motivation, safety, and the low cost of commercial devices are some of the benefits of using virtual reality for neurological rehabilitation (Forsberg et al. 2015; Gustavsson et al. 2021; Moan et al. 2021). Nonetheless, some undesired effects (e.g. headache, sickness, or nausea) (Massetti et al. 2018), as well as the difficulty of transferring the complex skills trained in virtual environments to the real world and the lack of ecological validity in a neurologically impaired population (Levac et al. 2019), were reported. Specifically, for balance training, the time of latency, the underestimation of perceived distances, and the dependence on specific systems (e.g. balance board) and virtual contexts were proposed as potential weaknesses of virtual reality environments (Morel et al. 2015).
Multiple sclerosis is a global neurodegenerative disease affecting approximately three million people in the world (Tafti et al. 2022). Balance disorders, gait impairments, and fatigue are the main symptoms in patients with multiple sclerosis that obtain positive effects with physical therapy intervention (Amedoro et al. 2020; Abou et al. 2022). Particularly, virtual reality-based physical rehabilitation showed benefits for balance and gait training (Casuso-Holgado et al. 2018; Nascimento et al. 2021); however, fatigue is a significant barrier to participation in physical activity, which influences the participants’ adherence (Moore et al. 2022). A recent systematic review has summarised dropout data from randomised control clinical trials about exercise interventions in people with multiple sclerosis, concluding that mean age, the proportion of females, and intervention duration were moderators inversely associated with adherence (Dennett et al. 2020). Therefore, these findings could impact the sample size calculation, promoting an under- or overestimation. Furthermore, this could influence the differential dropout rate, which is how the degree of dropout differs between the intervention and comparator conditions after randomisation (Crutzen et al. 2015). It might affect the power of research and could present a risk of bias for randomised control clinical trials (Cooper et al. 2018). In view of this background, setting accurate expected dropout rates in virtual reality studies for rehabilitation in multiple sclerosis could help future trials to avoid problems in their internal or external validity. In addition, the identification of factors specifically associated with dropout in virtual reality trials could help clinicians when translating research into practice.
As far as we are concerned, no previous systematic reviews were found reporting dropout in virtual reality interventions for balance and gait rehabilitation in this population. Thus, the present systematic review and meta-analysis aimed to: (1) systematically assess and meta-analyse the overall pooled dropout rate of randomised controlled trials using virtual reality as an intervention for balance or gait training in people with multiple sclerosis in both absolute and comparative terms; (2) analyse whether any participant or intervention factors are related to dropout; and (3) identify adverse events that could be the reason for dropouts.
2 Methods
2.1 Data sources and search strategy
This systematic review was carried out following the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al. 2009). The review protocol was registered in the PROSPERO database (Registration number: CRD42021284989).
Two independent reviewers (M.J.C.-H., C.G.-M.) conducted an electronic search in MEDLINE (PubMed), Scopus, Web of Science (WOS), the Physiotherapy Evidence Database (PEDro), the Cochrane Database of Systematic Reviews (CDSR), CINHAL, LILACS, ScienceDirect, and ProQuest. The search was performed between July and November 2021. Neither language nor date filters were applied in the different databases. Key terms concerning intervention (‘virtual reality’, ‘game’, ‘gaming’, ‘exergaming’, and ‘interactive’), balance (‘balance’ or ‘postural control’), gait (‘gait’, ‘walking’, and ‘ambulation’), and ‘multiple sclerosis’ were combined as search terms in the strategies. The search strategy is shown in detail in Supplemental Material 1.
2.2 Research question and study selection
The participants, interventions, comparisons, outcomes, and study design (PICOS) model was considered to set the following research questions: what dropout data are reported during the intervention and follow-up period by randomised control clinical trials conducting virtual reality intervention to improve balance or gait in multiple sclerosis and what are the possible moderators affecting dropout in these studies?
Participants included in the review were female or male, aged between 18 and 65 years old, with any diagnosis of multiple sclerosis phenotype meeting the revised McDonald criteria (Thompson et al. 2018). Walking ability was preserved according to the Expanded Disability Status Scale (EDSS) score (EDSS ≤ 6). Included interventions involved any type of virtual reality systems aimed at improving balance or gait compared to other interventions based on physical activity with or without external aid use. Furthermore, studies that reported dropout event information were included.
2.3 Data extraction and quality assessment
First, two independent reviewers (C.G.-M. and M.J.C.-H.) identified potential articles in databases to be included in the systematic review through the title and abstract information. Next, duplicates were removed, and an exhaustive analysis of articles was carried out based on their full-text reading. This step was particularly focussed on the selection criteria assessment, ensuring that the inclusion criteria were met before selecting suitable studies. In the case of disagreement, a third reviewer (M.-D.C.-V.) was consulted to decide on the inclusion of the documents.
Once articles were selected, the quality assessment was conducted using the PEDro scale (Maher et al. 2003) and the Revised Cochrane risk-of-bias tool for randomised trials (RoB-2) (Higgins et al. 2019). PEDro is a reliable tool of 11 items that evaluates the inner validity of a clinical trial. If studies score above 6 points, they are classified as level I evidence (6–8: good; 8–10: excellent). If the score is below 5, they are classified as level II (4–5: deficient; < 4: poor). ROB-2 allows the evaluation of bias in randomised control trials, comprising five domains (bias arising from the randomisation process, due to deviations from the intended interventions, to missing outcome data, in the measurement of the outcome, and in the selection of the reported result) that are qualified as a low or high risk of bias with some concerns (Sterne et al. 2019).
Next, reviewers recorded the data for qualitative and quantitative synthesis. The extracted data were country, multiple sclerosis phenotype and disability status, female and male percentages, age, experimental and comparator group intervention characteristics, number of participants recruited and analysed, retention rate, dropout rates (for the experimental and control groups), reasons for dropout (in each group), and adverse events. Disagreements in data were solved by consensus with a third reviewer. Information provided by the included studies allowed us to calculate dropout rates in all cases, so no corresponding authors were contacted.
2.4 Data analysis
Dropout rate was calculated as the number of participants who did not complete the intervention and follow-up period divided by the total number of participants that underwent the randomisation process. Moreover, retention rate was the total number of participants that concluded the intervention, showing the adherence rate to treatment. For those studies that included more than two groups of intervention, comparison between groups was analysed separately two by two.
To conduct the meta-analysis, the R Studio software (version 4.0.0) and its packages meta, metafor, and dmetar were used (Viechtbauer 2010; Balduzzi et al. 2019; Harrer et al. 2021). The proportion meta-analysis was performed through the metaprop function to determine the estimated dropout rate in virtual reality intervention, the control comparator, and all arms. Proportions were transformed using the logit transformation (Schwarzer et al. 2019).
A binary meta-analysis based on odds ratios (ORs) was conducted to examine whether the probability of dropouts is higher in the virtual reality or in the comparator interventions. To assess the effect measure in binary outcomes, the OR with a 95% confidence interval (95%CI) was calculated, and the inverse variance method was used to adjust pooling estimations to sparse data (considering that dropouts are a rare event). Likewise, the Hartung–Knapp adjustment for a random effects model was implemented. Focussing on ORs, if the value is 1, there are no differences in dropouts between the experimental and comparator groups. In contrast, if the OR is greater than 1, a higher dropout rate was registered for the experimental group. The restricted maximum-likelihood estimator for tau2 was selected to estimate the between-study variance (Viechtbauer 2005). As some studies could present zero events in the experimental and/or comparator arm, a 0.5 continuity correction was added to all meta-analyses, as suggested by Gart and Zweifel (1967).
Heterogeneity between studies was assessed through I2, tau2, and Cochrane’s Q (p < 0.05 indicates heterogeneity). When I2 presents a value above 50%, it means that large heterogeneity is found across studies (Higgins et al. 2021). A random effects model was employed considering the possible degree of heterogeneity between the included studies.
Forest plots were used to show the outcomes of proportions and binary meta-analyses. The prediction interval was added as a red line to the forest plot to provide a measure of reliability of future treatment effects in new studies (Nagashima et al. 2019). Depending on the level of immersion of the subject within the virtual environment, virtual reality was classified as non-immersive, semi-immersive, and fully immersive for subgroup analysis.
A sensitivity analysis was carried out to assess the influence of studies on the overall binary meta-analysis results. The influence was explored to detect the presence of outlier data and whether there were studies that contributed to heterogeneity or bias pooled results. A Baujat plot, a L’Abbé plot, and influence graphs were created to represent influential cases in meta-analysis. The influence graphs showed the studies that significantly influenced the pooled effect size in red. In addition, an exploratory graphical analysis of data was performed to examine whether there is a clear trend of effect size related to independent variables.
Meta-regression was conducted to evaluate possible associations between participants or study characteristics which could vary in the presence of dropout events. Studies with no available data were excluded from the meta-regression analysis. Moreover, to run the meta-regression, at least three studies with the predictor were needed. The analysed moderators were interventions, number, duration, frequency and weeks of sessions, EDSS score, multiple sclerosis phenotype, and sex.
Publication bias and small study effects were evaluated through a contour-enhanced funnel plot adjusted by the Duval and Tweedie trim and fill method (Shi and Lin 2020). Asymmetry in the funnel plot indicated the effect of small studies in the pooled results. To confirm the absence of asymmetry, a p value greater than 0.05 must be reached in the Harbord’s test (Harbord et al. 2006) and the Egger bias test (Egger et al. 1997).
3 Results
3.1 Study selection and methodological quality assessment
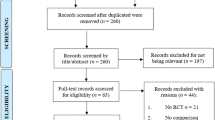
In total, 7024 articles were identified through the initial database search based on titles and abstracts. After that, duplicates were removed, obtaining 5995 articles. Once the studies underwent the screening and eligibility steps, 16 randomised control trials were included for the qualitative synthesis and quantitative analysis. There was no disagreement between reviewers in the study selection process. Figure 1 showed the PRISMA flowchart detailing the selection procedure. Excluded studies and their reasons are detailed in Supplemental Material 2.
Flow diagram of trials selection based on PRISMA 2020 guidelines. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools. From: Page et al. (2021). For more information, visit: http://www.prisma-statement.org/
Regarding the quality assessments, the PEDro scale results are shown in Supplemental Material 3. PEDro scores were reported from the included studies: thirteen with level I evidence (Lozano-Quilis et al. 2014; Hoang et al. 2016; Kalron et al. 2016; Calabrò et al. 2017; Peruzzi et al. 2017; Russo et al. 2018; Khalil et al. 2019; Munari et al. 2020; Ozkul et al. 2020; Tollar et al. 2020; Molhemi et al. 2021, 2022; Pagliari et al. 2021) and three with level II (Brichetto et al. 2015; Robinson et al. 2015; Yazgan et al. 2020). Most studies were single blinded, with the assessor being blinded to participant allocation. In addition, the ROB-2 overall score reported that most studies presented some concerns, but only three studies (Robinson et al. 2015; Ozkul et al. 2020; Yazgan et al. 2020) had a ‘high risk’ of bias (Fig. 2). Disagreements between reviewers occasionally occurred for domain 2, but consensus was always reached without the participation of the third reviewer.
3.2 Study design and population characteristics
The main characteristics of the participants and the interventions are shown in Table 1. The randomised pooled population obtained from the reviewed studies reached a total of 656 participants with a mean EDSS score of 4.22 (95%CI 4.15–4.30). The mean age was 45.12 (95%CI 44.66–45.59), and 65.57% of the population were female. All studies involved patients with relapsing–remitting type, except for three studies which did not specify the phenotype of multiple sclerosis (Robinson et al. 2015; Kalron et al. 2016; Pagliari et al. 2021). Furthermore, eight studies (Lozano-Quilis et al. 2014; Brichetto et al. 2015; Hoang et al. 2016; Munari et al. 2020; Tollar et al. 2020; Yazgan et al. 2020; Molhemi et al. 2021, 2022) involved participants with any type of multiple sclerosis (relapsing–remitting, secondary progressive, and primary progressive) without subgroup analysis.
Concerning the immersion of the virtual reality systems, 14 studies employed non-immersive virtual reality as the main experimental intervention and four of them used the Wii Fit system (Brichetto et al. 2015; Robinson et al. 2015; Khalil et al. 2019; Yazgan et al. 2020). Only two trials used fully immersive virtual reality (Kalron et al. 2016; Ozkul et al. 2020).
Most studies compared the virtual reality intervention to improve balance or gait to conventional balance training (n = 13, 81.25%) (Lozano-Quilis et al. 2014; Brichetto et al. 2015; Robinson et al. 2015; Hoang et al. 2016; Kalron et al. 2016; Peruzzi et al. 2016; Calabrò et al. 2017; Russo et al. 2018; Khalil et al. 2019; Ozkul et al. 2020; Molhemi et al. 2021, 2022; Pagliari et al. 2021), followed by robotic-assisted gait training (n = 3, 18.75%) (Calabrò et al. 2017; Peruzzi et al. 2017; Munari et al. 2020). The lowest number of sessions performed was 8 (Robinson et al. 2015), while the highest was 54 (Russo et al. 2018). Most authors proposed a frequency of intervention of 2 times per week with a minimum time per session of 30 min (Hoang et al. 2016; Kalron et al. 2016) and a maximum of 85 min (Calabrò et al. 2017).
The mean number of dropout events for the experimental group was 1.61 cases and 1.88 for the comparator group. The highest number of dropouts in the virtual reality groups was registered by Hoang et al. (2016) and Pagliari et al. (2021). The reasons reported by the authors for dropout in both groups were: difficulties reaching the research centre, transportation problems, scheduling problems, moving to another city, refusal to participate, personal or familial issues, lack of motivation or time, loss of data due to administrative problems, exacerbation of symptoms, disease relapse, work intensity, and illness/medical reasons/hospitalisation not related to multiple sclerosis. Three studies did not report any dropout events during the intervention or follow-up period (Brichetto et al. 2015; Calabrò et al. 2017; Russo et al. 2018).
3.3 Meta-analysis of proportions
A total of 18 arms (k) from 16 studies were included in the proportion and binary meta-analysis, since one of the randomised control trials presented three study groups (Tollar et al. 2020). From a total of 638 participants, 63 cases of dropouts were reported. The forest plot showed an overall pooled dropout rate of 6.6% (95%CI 3.2–12.9%) without heterogeneity between studies (tau2 = 1.18, Q = 10.07, df = 17, I2 = 0%, 95%CI 0–50%, p = 0.90) (Fig. 3). The dropout rate for the virtual reality-based interventions was 5.7% (95%CI 2.3–13.6%) against the 9.7% (95%CI 5.7–16.02%) in the comparator groups (Supplemental Material 4). Conversely, the retention rate for the virtual reality and comparator groups was 94.3% and 90.3%, respectively. None of the prediction intervals calculated across the meta-analysis suggested that the intervention would achieve the same effects in the future.
3.4 Binary meta-analysis (OR)
The main results showed a slightly lower probability that dropouts occurred in the virtual reality-based interventions than in the comparator groups, but a significant difference was not obtained (OR = 0.89, 95%CI 0.64–1.24, p = 0.46). No significant heterogeneity between studies was found (tau2 = 0, Q = 5.6, df = 17, I2 = 0%, 95%CI 0–50%, p = 0.99) (Fig. 4). The prediction interval confirmed that the same effects would not happen in the future studies. A subgroup meta-analysis according to the immersion level of the virtual reality was not carried out because the number of studies using immersive systems did not reach the minimum required (3 studies).
A post hoc sensitive analysis using the L’Abbé and Baujat plots and influence graphs (Supplemental Material 5) showed that none of the included studies influenced heterogeneity or bias for the pooled effect size, and no outliers were found. Additionally, no small study effects or publication bias was shown in the contour-enhanced funnel plot (Fig. 5), the Harbord test (p = 0.37), or the Egger bias test (p = 0.34).
3.5 Meta-regression
The meta-regression revealed that the type of intervention, number, frequency, and duration of session, weeks of intervention, EDSS score, multiple sclerosis phenotype, sex, and methodological quality could not be related to the dropout events. A detailed description of the analysis is shown in Table 2.
4 Discussion
A total of 16 randomised control trials reporting dropouts were meta-analysed to calculate the overall pooled dropout rate of virtual reality-based interventions for the improvement of balance and gait in patients with multiple sclerosis. The main clinical implication of the results of our study was that the virtual reality-based training for balance and gait in people with multiple sclerosis was highly accepted with a low dropout rate and high adherence during the study period. Torous et al. (2020) suggested that the retention in research contexts could change when experimental approaches are translated into a clinical setting. This could be especially important for long rehabilitation programmes in chronic conditions. A recent study (Hortobágyi et al. 2022) reported a high adherence rate to a two-year maintenance programme including exergaming in people with multiple sclerosis; however, the sample size was very small, and more research about long-term adherence to virtual reality rehabilitation in this population is needed.
Adherence is one of the main conflicts faced in rehabilitation; the therapeutic approach of multiple sclerosis is not an exception. As a result, looking for rehabilitation therapies that achieve higher participant compliance to treatment is vital (Arafah et al. 2017). If correct adherence is not achieved, the effectiveness of the rehabilitation might be limited and incur additional healthcare costs (Jack et al. 2010; Room et al. 2021). Accordingly, the previous literature has proposed that virtual reality strategies presented higher adherence in patients with neurological disorders (Asadzadeh et al. 2021; Dalmazane et al. 2021). Nonetheless, our results suggested lower dropout rates in virtual reality-based interventions, which may be confirmed with larger sample sizes. This idea is supported by the prediction intervals, which stated that our findings could change with future trials. The recent systematic review of Bevens et al. (2021) analysed the dropout rate in people with multiple sclerosis who received digital health interventions, showing no significant differences between experimental and control comparators. Therefore, we can consider that the adherence to virtual reality or other technological approaches were at least similar to other interventions.
During the screening process, several studies were discarded because dropouts were not mentioned. Despite CONSORT guidelines stating the need to report complete data, many authors do not know how to handle dropouts (Bell et al. 2013). To address this issue, it is necessary to standardise the way in which the reason and number of dropouts are described, for example, using the CONSORT flowchart of the study period. Also, further details of dropouts could help to make decisions regarding which interventions to offer to whom (Wright et al. 2021).
Our meta-regression data showed that the type of intervention, number, duration, and frequency of sessions, weeks of intervention, disability score, phenotype, sex, and methodological quality were not predictors of dropouts. Although it seems that a higher frequency of sessions could favour participant dropouts, no significant results were found. Similar results were obtained by Dennett et al. (2020), who stated that there was no relationship between the frequency of exercise-based sessions and dropouts, but duration modified the likelihood of dropouts. Although our protocol included the analysis according to the level of immersion, fully immersive and semi-immersive virtual reality was excluded from the moderator analysis because of the limited number of studies included. Therefore, we suggest to provide a specific dropout rate analysis when the proportion of studies using immersive virtual reality rises, since higher immersion and presence levels are expected to achieve a higher treatment adherence (Rose et al. 2018; Dębska et al. 2019). Additionally, future studies should evaluate enjoyment and motivation with specific measurement scales, allowing researchers to understand whether motivation or enjoyment during the intervention is predictors of dropout or adherence to treatment in the targeted population.
According to the literature (Grover et al. 2021), adverse events due to treatment are considered one of the main causes of dropouts. Nonetheless, we were unable to analyse them as a moderator of dropout rate, since none of the studies included reported the undesired effects of the virtual reality intervention. Two possible explanations behind the low number of studies describing adverse events or side effects because of the intervention were considered: the first is that participants did not actually have adverse effects due to the virtual reality-based intervention, and the second is that the authors decided not to report them. The latter idea is supported by Phillips et al. (2019) and Pitrou et al. (2009), who addressed methodological weaknesses in reporting adverse events in randomised control trials, leading to a misinterpretation of intervention safety.
4.1 Strength and limitations
This is the first meta-analysis to calculate the overall pooled dropout rate for innovative virtual reality-based interventions in patients with multiple sclerosis. The findings of this review could help future randomised control trials to calculate their sample size to avoid dropout bias. Furthermore, no heterogeneity between the included studies was found in the analysis. The sensitivity analysis did not report any randomised control trial as an outlier that could strongly influence the overall size effect. Moreover, the funnel plot did not show any publication bias.
The main limitation of this review was the small sample size that the randomised control trials included, so a larger overall sample size would make our results more reliable. Another issue was that many studies did not report detailed reasons for dropouts. Furthermore, adverse events were not reported, so it was not possible to determine whether they could be moderators for dropout rate.
5 Conclusion
The overall pooled dropout rate of randomised control trials on virtual reality for balance or gait training in people with multiple sclerosis was 6.6%. Our analysis reported no differences in dropout rate for participants who received virtual reality-based interventions versus other comparators; however, the lower dropout rate in the virtual reality group could indicate that the inclusion of larger sample sizes would show a significant difference in favour of the virtual reality group. The number, duration, frequency, and weeks of sessions, sex, age, phenotype, disability, and methodological quality were not determined to be moderators of dropouts. Adverse events were not reported by the studies included, making it impossible to analyse their influence as moderators.
Future randomised control trials should standardise the description of dropout causes and adverse effects of the rehabilitation treatments. Furthermore, the advantages of virtual reality, such as motivation and enjoyment, should be systematically assessed in clinical trials to determine whether these outcomes are indeed moderators of dropout and adherence.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
Abou L, Qin K, Alluri A, Yitin D, Laura A (2022) The effectiveness of physical therapy interventions in reducing falls among people with multiple sclerosis: a systematic review and meta-analysis. J Bodyw Mov Ther 29:74–85. https://doi.org/10.1016/j.jbmt.2021.09.015
Amedoro A, Berardi A, Conte A, Donatella V, Giuseppe M, Marco T, Giovanni G (2020) The effect of aquatic physical therapy on patients with multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord 41:102022. https://doi.org/10.1016/j.msard.2020.102022
Arafah AM, Bouchard V, Mayo NE (2017) Enrolling and keeping participants in multiple sclerosis self-management interventions: a systematic review and meta-analysis. Clin Rehabil 31:809–823. https://doi.org/10.1177/0269215516658338
Asadzadeh A, Samad-Soltani T, Salahzadeh Z, Rezaei-Hachesu P (2021) Effectiveness of virtual reality-based exercise therapy in rehabilitation: a scoping review. Inform Med Unlocked 24:100562. https://doi.org/10.1016/j.imu.2021.100562
Balduzzi S, Rücker G, Schwarzer G (2019) How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Heal 22:153–160. https://doi.org/10.1136/ebmental-2019-300117
Bell ML, Kenward MG, Fairclough DL, Horton NJ (2013) Differential dropout and bias in randomised controlled trials: when it matters and when it may not. BMJ 346:e8668–e8668. https://doi.org/10.1136/bmj.e8668
Bevens W, Gray K, Jelinek GA, Tracey W, Steve S (2021) Attrition within digital health interventions for people with multiple sclerosis: systematic review and meta-analysis. J Med Internet Res. https://doi.org/10.2196/27735
Brichetto G, Piccardo E, Pedullà L, Battaglia MA, Tacchino A (2015) Tailored balance exercises on people with multiple sclerosis: a pilot randomized, controlled study. Mult Scler 21:1055–1063. https://doi.org/10.1177/1352458514557985
Calabrò RS, Russo M, Naro A, De Luca R, Leo A, Tomasello P, Molonia F, Dattola V, Bramanti A, Bramanti P (2017) Robotic gait training in multiple sclerosis rehabilitation: can virtual reality make the difference? Findings from a randomized controlled trial. J Neurol Sci 377:25–30. https://doi.org/10.1016/J.JNS.2017.03.047
Casuso-Holgado MJ, Martín-Valero R, Carazo AF, Medrano-Sanchez E, Dolores Cortes-Vega M, Jose Montero-Bancalero F (2018) Effectiveness of virtual reality training for balance and gait rehabilitation in people with multiple sclerosis: a systematic review and meta-analysis. Clin Rehabil. https://doi.org/10.1177/0269215518768084
Cooper CL, Whitehead A, Pottrill E, Julious SA, Walters S (2018) Are pilot trials useful for predicting randomisation and attrition rates in definitive studies: a review of publicly funded trials. Clin Trials 15:189–196. https://doi.org/10.1177/1740774517752113
Crutzen R, Viechtbauer W, Spigt M, Kotz D (2015) Differential attrition in health behaviour change trials: a systematic review and meta-analysis. Psychol Heal 30:122–134. https://doi.org/10.1080/08870446.2014.953526
Dalmazane M, Gallou-Guyot M, Compagnat M, Magy L, Montcuquet A, Billot M, Daviet JC, Perrochon A (2021) Effects on gait and balance of home-based active video game interventions in persons with multiple sclerosis: a systematic review. Mult Scler Relat Disord 51:102928. https://doi.org/10.1016/j.msard.2021.102928
Dębska M, Polechoński J, Mynarski A, Polechoński P (2019) Enjoyment and intensity of physical activity in immersive virtual reality performed on innovative training devices in compliance with recommendations for health. Int J Environ Res Public Health 16:3673. https://doi.org/10.3390/ijerph16193673
Dennett R, Madsen LT, Connolly L, Hosking J, Dalgas U, Freeman J (2020) Adherence and drop-out in randomized controlled trials of exercise interventions in people with multiple sclerosis: a systematic review and meta-analyses. Mult Scler Relat Disord 43:102169. https://doi.org/10.1016/j.msard.2020.102169
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. https://doi.org/10.1136/bmj.315.7109.629
Forsberg A, Nilsaga˚rd Y, Boström K, (2015) Perceptions of using videogames in rehabilitation: a dual perspective of people with multiple sclerosis and physiotherapists. Disabil Rehabil 37:338–344. https://doi.org/10.3109/09638288.2014.918196
Gart JJ, Zweifel JR (1967) On the bias of various estimators of the logit and its variance with application to quantal bioassay. Biometrika 54:181–187. https://doi.org/10.2307/2333861
Grover S, Mallnaik S, Chakrabarti S, Mehra A (2021) Factors associated with dropout from treatment: An exploratory study. Indian J Psychiatry 63:41. https://doi.org/10.4103/psychiatry.IndianJPsychiatry_87_19
Gustavsson M, Kjörk EK, Erhardsson M, Alt Murphy M (2021) Virtual reality gaming in rehabilitation after stroke–user experiences and perceptions. Disabil Rehabil. https://doi.org/10.1080/09638288.2021.1972351
Harbord RM, Egger M, Sterne JAC (2006) A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 25:3443–3457. https://doi.org/10.1002/sim.2380
Harrer M, Cuijpers P, Furukawa TA, Ebert DD (2021) Doing meta-analysis with R. Chapman and Hall/CRC, New York
Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (2021) Cochrane Handbook for Systematic Reviews of Interventions version 6.2. The Cochrane Collaboration. Wiley, Chichester
Higgins JP, Savović J, Page MJ, Sterne JAC (2019) Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) Full Guidance Document. Br Med J 1–72
Hoang P, Schoene D, Gandevia S, Smith S, Lord SR (2016) Effects of a home-based step training programme on balance, stepping, cognition and functional performance in people with multiple sclerosis—a randomized controlled trial. Mult Scler J 22:94–103. https://doi.org/10.1177/1352458515579442
Hortobágyi T, Ács P, Baumann P, Borbély G, Afra G, Reichardt-Varga E, Sántha G, Tollár J (2022) Comparative effectiveness of 4 exercise interventions followed by 2 years of exercise maintenance in multiple sclerosis: a randomized controlled trial. Arch Phys Med Rehabil. https://doi.org/10.1016/j.apmr.2022.04.012
Jack K, McLean SM, Moffett JK, Gardiner E (2010) Barriers to treatment adherence in physiotherapy outpatient clinics: a systematic review. Man Ther 15:220–228. https://doi.org/10.1016/j.math.2009.12.004
Kalron A, Fonkatz I, Frid L, Baransi H, Achiron A (2016) The effect of balance training on postural control in people with multiple sclerosis using the CAREN virtual reality system: a pilot randomized controlled trial. J Neuroeng Rehabil 13:13. https://doi.org/10.1186/s12984-016-0124-y
Khalil H, Al-Sharman A, El-Salem K, Alghwiri A, Al-Shorafat D, Khazaaleh S, Abu foul L, (2019) The development and pilot evaluation of virtual reality balance scenarios in people with multiple sclerosis (MS): a feasibility study. NeuroRehabilitation 43:473–482. https://doi.org/10.3233/NRE-182471
Levac DE, Huber ME, Sternad D (2019) Learning and transfer of complex motor skills in virtual reality: a perspective review. J Neuroeng Rehabil 16:1–15. https://doi.org/10.1186/s12984-019-0587-8
Lozano-Quilis J-A, Gil-Gómez H, Gil-Gómez J-A, Albiol-Pérez S, Palacios-Navarro G, Fardoun HM, Mashat AS (2014) Virtual rehabilitation for multiple sclerosis using a kinect-based system: randomized controlled trial. JMIR Serious Games 2:e12. https://doi.org/10.2196/games.2933
Maher CG, Sherrington C, Herbert RD (2003) Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 83:713–721. https://doi.org/10.1093/ptj/83.8.713
Massetti T, Dias T, Crocetta TB, Guarnieri R, de Freitas BL, Bianchi Lopes P, Watson S, Tonks J, de Mello Monteiro CB (2018) The clinical utility of virtual reality in neurorehabilitation: a systematic review. J Cent Nerv Syst Dis. https://doi.org/10.1177/1179573518813541
Matamala-Gomez M, Bottiroli S, Realdon O, Riva G, Galvagni L, Platz T, Sandrini G, De Icco R, Tassorelli C (2021) Telemedicine and virtual reality at time of COVID-19 pandemic: an overview for future perspectives in neurorehabilitation. Front Neurol 12:1–9. https://doi.org/10.3389/fneur.2021.646902
Moan ME, Vonstad EK, Su X, Vereijken B, Solbjør M, Skjæret-Maroni N (2021) Experiences of stroke survivors and clinicians with a fully immersive virtual reality treadmill exergame for stroke rehabilitation: a qualitative pilot study. Front Aging Neurosci 13:1–12. https://doi.org/10.3389/fnagi.2021.735251
Moher D, Liberatu A, Tetzlaff j, Altman D, (2009) Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. Ann Intern Med 151:264. https://doi.org/10.7326/0003-4819-151-4-200908180-00135
Molhemi F, Monjezi S, Mehravar M, Shaterzadeh-Yazdi MJ, Salehi R, Hesam S, Mohammadianinejad E (2021) Effects of virtual reality vs conventional balance training on balance and falls in people with multiple sclerosis: a randomized controlled trial. Arch Phys Med Rehabil 102:290–299. https://doi.org/10.1016/j.apmr.2020.09.395
Molhemi F, Mehravar M, Monjezi S, Monjezi S, Salehi R, Negahban H, Shaterzadeh-Yazdi MJ, Majdinasab N (2022) Effects of exergaming on cognition, lower limb functional coordination, and stepping time in people with multiple sclerosis: a randomized controlled trial. Disabil Rehabil. https://doi.org/10.1080/09638288.2022.2060332
Moore H, Nair KPS, Baster K, Middleton R, Paling D, Sharrack B (2022) Fatigue in multiple sclerosis: a UK MS-register based study. Mult Scler Relat Disord 64:103954. https://doi.org/10.1016/j.msard.2022.103954
Morel M, Bideau B, Lardy J, Kulpa R (2015) Advantages and limitations of virtual reality for balance assessment and rehabilitation. Neurophysiol Clin 45:315–326. https://doi.org/10.1016/j.neucli.2015.09.007
Munari D, Fonte C, Varalta V, Battistuzzi E, Cassini S, Montagnoli AP, Gandolfi M, Modenese A, Filippetti M, Smania N, Picelli A (2020) Effects of robot-assisted gait training combined with virtual reality on motor and cognitive functions in patients with multiple sclerosis: a pilot, single-blind, randomized controlled trial. Restor Neurol Neurosci 38:151–164. https://doi.org/10.3233/RNN-190974
Nagashima K, Noma H, Furukawa TA (2019) Prediction intervals for random-effects meta-analysis: a confidence distribution approach. Stat Methods Med Res 28:1689–1702. https://doi.org/10.1177/0962280218773520
Nascimento AS, Fagundes CV, dos Mendes FAS, Leal CV (2021) Effectiveness of virtual reality rehabilitation in persons with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Mult Scler Relat Disord. https://doi.org/10.1016/j.msard.2021.103128
Ozkul C, Guclu-Gunduz A, Yazici G, Atalay Guzel N, Irkec C (2020) Effect of immersive virtual reality on balance, mobility, and fatigue in patients with multiple sclerosis: a single-blinded randomized controlled trial. Eur J Integr Med 35:101092. https://doi.org/10.1016/j.eujim.2020.101092
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:171
Pagliari C, Di Tella S, Jonsdottir J, Mendozzi L, Rovaris M, De Icco R, Milanesi T, Federico S, Agostini M, Goffredo M, Pellicciari L, Franceschini M, Cimino V, Bramanti P, Baglio F (2021) Effects of home-based virtual reality telerehabilitation system in people with multiple sclerosis: a randomized controlled trial. J Telemed Telecare. https://doi.org/10.1177/1357633X211054839
Peruzzi A, Cereatti A, Della Croce U, Mirelman A (2016) Effects of a virtual reality and treadmill training on gait of subjects with multiple sclerosis: a pilot study. Mult Scler Relat Disord 5:91–96. https://doi.org/10.1016/j.msard.2015.11.002
Peruzzi A, Zarbo IR, Cereatti A, Dela Croce U, Mirelman A (2017) An innovative training program based on virtual reality and treadmill: effects on gait of persons with multiple sclerosis. Disabil Rehabil 39:1557–1563. https://doi.org/10.1080/09638288.2016.1224935
Phillips R, Hazell L, Sauzet O, Cornelius V (2019) Analysis and reporting of adverse events in randomised controlled trials: a review. BMJ Open 9:e024537. https://doi.org/10.1136/bmjopen-2018-024537
Pitrou I, Boutron I, Ahmad N, Ravaud P (2009) Reporting of safety results in published reports of randomized controlled trials. Arch Intern Med 169:1756–1761. https://doi.org/10.1001/archinternmed.2009.306
Robinson J, Dixon J, Macsween A, van Schaik P, Martin D (2015) The effects of exergaming on balance, gait, technology acceptance and flow experience in people with multiple sclerosis: a randomized controlled trial. BMC Sports Sci Med Rehabil 7:8. https://doi.org/10.1186/s13102-015-0001-1
Room J, Boulton M, Dawes H, Archer K, Barker K (2021) Physiotherapists’ perceptions of how patient adherence and non-adherence to recommended exercise for musculoskeletal conditions affects their practice: a qualitative study. Physiotherapy 113:107–115. https://doi.org/10.1016/j.physio.2021.06.001
Rose T, Nam CS, Chen KB (2018) Immersion of virtual reality for rehabilitation—review. Appl Ergon 69:153–161. https://doi.org/10.1016/j.apergo.2018.01.009
Russo M, Dattola V, De Cola MC, Logiudice AL, Porcari B, Cannavò A, Sciarrone F, De Luca R, Molonia F, Sessa E, Bramanti P, Calabrò RS (2018) The role of robotic gait training coupled with virtual reality in boosting the rehabilitative outcomes in patients with multiple sclerosis. Int J Rehabil Res 41:166–172. https://doi.org/10.1097/MRR.0000000000000270
Schwarzer G, Chemaitelly H, Abu-Raddad LJ, Rücker G (2019) Seriously misleading results using inverse of Freeman–Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods 10:476–483. https://doi.org/10.1002/jrsm.1348
Shi L, Lin L (2020) The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine (baltimore) 98:e15987. https://doi.org/10.1097/MD.0000000000015987
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. https://doi.org/10.1136/bmj.l4898
Tafti D, Ehsan M, Xixis KL (2022) Multiple Sclerosis. StatPearls Publishing, Treasure Island (FL)
Thompson AJ, Banwell BL, Barkhof F, Carroll CT, Comi C, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintoré M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173. https://doi.org/10.1016/S1474-4422(17)30470-2
Tollar J, Nagy F, Toth BE, Torok K, Szita K, Csutoras B, Moizs M, Hortobagyi T (2020) Exercise effects on multiple sclerosis quality of life and clinical-motor symptoms. Med Sci Sports Exerc 52:1007–1014. https://doi.org/10.1249/MSS.0000000000002228
Torous J, Lipschitz J, Ng M, Firth J (2020) Dropout rates in clinical trials of smartphone apps for depressive symptoms: a systematic review and meta-analysis. J Affect Disord 263:413–419. https://doi.org/10.1016/j.jad.2019.11.167
Viechtbauer W (2005) Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat 30:261–293. https://doi.org/10.3102/10769986030003261
Viechtbauer W (2010) Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. https://doi.org/10.18637/jss.v036.i03
Voinescu A, Sui J, Stanton Fraser D (2021) Virtual reality in neurorehabilitation: an umbrella review of meta-analyses. J Clin Med. https://doi.org/10.3390/jcm10071478
Wright I, Mughal F, Bowers G, Meiser-Stedman R (2021) Dropout from randomised controlled trials of psychological treatments for depression in children and youth: a systematic review and meta-analyses. J Affect Disord 281:880–890. https://doi.org/10.1016/j.jad.2020.11.039
Yazgan YZ, Tarakci E, Tarakci D, Ozdincler AR, Kurtuncu M (2020) Comparison of the effects of two different exergaming systems on balance, functionality, fatigue, and quality of life in people with multiple sclerosis: a randomized controlled trial. Mult Scler Relat Disord 39:101902. https://doi.org/10.1016/j.msard.2019.101902
Author information
Authors and Affiliations
Contributions
MJC-H and CG-M were involved in conceptualisation and writing, review and editing; CG-M, MJC-H contributed to methodology; CG-M were involved in software and formal analysis; MJC-H, CG-M, MDC-V, RM-V, JAM-M and DL-A contributed to writing—original draft preparation; MDC-V and R-MV were involved in visualisation; MDC-V, JAM-M and DL-A contributed to supervision; MJC-H and CG-M contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Casuso-Holgado, M.J., García-Muñoz, C., Martín-Valero, R. et al. Dropout rate in randomised controlled trials of balance and gait rehabilitation in multiple sclerosis: is it expected to be different for virtual reality-based interventions? A systematic review with meta-analysis and meta-regression. Virtual Reality 27, 3451–3467 (2023). https://doi.org/10.1007/s10055-022-00733-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10055-022-00733-4