Abstract
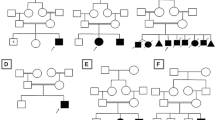
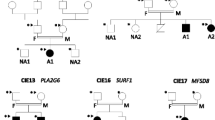
Cerebellar and/or vermis atrophy is recognized in various types of childhood disorders with clinical and genetic heterogeneity. Although careful evaluation of clinical features and neuroimaging can lead to correct diagnosis of disorders, their diagnosis is sometimes difficult because clinical features can overlap with each other. In this study, we performed family-based whole exome sequencing of 23 families including 25 patients with cerebellar and/or vermis atrophy in childhood, who were unable to be diagnosed solely by clinical examination. Pathological mutations of seven genes were found in ten patients from nine families (9/23, 39.1 %): compound heterozygous mutations in FOLR1, C5orf42, POLG, TPP1, PEX16, and de novo mutations in CACNA1A, and ITPR1. Patient 1A with FOLR1 mutations showed extremely low concentration of 5-methyltetrahydrofolate in the cerebrospinal fluid and serum, and Patient 6 with TPP1 mutations demonstrated markedly lowered tripeptidyl peptidase 1 activity in leukocytes. Furthermore, Patient 8 with PEX16 mutations presented a mild increase of very long chain fatty acids in the serum as supportive data for genetic diagnosis. The main clinical features of these ten patients were nonspecific and mixed, and included developmental delay, intellectual disability, ataxia, hypotonia, and epilepsy. Brain MRI revealed both cerebellar and vermis atrophy in eight patients (8/10, 80 %), vermis atrophy/hypoplasia in two patients (2/10, 20 %), and brainstem atrophy in one patient (1/10, 10 %). Our data clearly demonstrate the utility of whole exome sequencing for genetic diagnosis of childhood cerebellar and/or vermis atrophy.


Similar content being viewed by others
References
Poretti A, Wolf NI, Boltshauser E (2008) Differential diagnosis of cerebellar atrophy in childhood. Eur J Paediatr Neurol 12(3):155–167. doi:10.1016/j.ejpn.2007.07.010
Al-Maawali A, Blaser S, Yoon G (2012) Diagnostic approach to childhood-onset cerebellar atrophy: a 10-year retrospective study of 300 patients. J Child Neurol 27(9):1121–1132. doi:10.1177/0883073812448680
Schaefer AM, McFarland R, Blakely EL, He L, Whittaker RG, Taylor RW, Chinnery PF, Turnbull DM (2008) Prevalence of mitochondrial DNA disease in adults. Ann Neurol 63(1):35–39. doi:10.1002/ana.21217
Santorelli FM, Garavaglia B, Cardona F, Nardocci N, Bernardina BD, Sartori S, Suppiej A, Bertini E, Claps D, Battini R, Biancheri R, Filocamo M, Pezzini F, Simonati A (2013) Molecular epidemiology of childhood neuronal ceroid-lipofuscinosis in Italy. Orphanet J Rare Dis 8:19. doi:10.1186/1750-1172-8-19
Erichsen AK, Koht J, Stray-Pedersen A, Abdelnoor M, Tallaksen CM (2009) Prevalence of hereditary ataxia and spastic paraplegia in southeast Norway: a population-based study. Brain 132(Pt 6):1577–1588. doi:10.1093/brain/awp056
Matilla-Duenas A (2012) The ever expanding spinocerebellar ataxias. Editorial Cerebellum 11(4):821–827. doi:10.1007/s12311-012-0376-4
Valente EM, Brancati F, Boltshauser E, Dallapiccola B (2013) Clinical utility gene card for: Joubert syndrome—update 2013. Eur J Hum Genet. doi:10.1038/ejhg.2013.10
Vermeer S, van de Warrenburg BP, Willemsen MA, Cluitmans M, Scheffer H, Kremer BP, Knoers NV (2011) Autosomal recessive cerebellar ataxias: the current state of affairs. J Med Genet 48(10):651–659. doi:10.1136/jmedgenet-2011-100210
Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, Shendure J (2011) Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet 12(11):745–755. doi:10.1038/nrg3031
Vissers LE, de Ligt J, Gilissen C, Janssen I, Steehouwer M, de Vries P, van Lier B, Arts P, Wieskamp N, del Rosario M, van Bon BW, Hoischen A, de Vries BB, Brunner HG, Veltman JA (2010) A de novo paradigm for mental retardation. Nat Genet 42(12):1109–1112. doi:10.1038/ng.712
de Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T, Vulto-van Silfhout AT, Koolen DA, de Vries P, Gilissen C, del Rosario M, Hoischen A, Scheffer H, de Vries BB, Brunner HG, Veltman JA, Vissers LE (2012) Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med 367(20):1921–1929. doi:10.1056/NEJMoa1206524
Saitsu H, Nishimura T, Muramatsu K, Kodera H, Kumada S, Sugai K, Kasai-Yoshida E, Sawaura N, Nishida H, Hoshino A, Ryujin F, Yoshioka S, Nishiyama K, Kondo Y, Tsurusaki Y, Nakashima M, Miyake N, Arakawa H, Kato M, Mizushima N, Matsumoto N (2013) De novo mutations in the autophagy gene WDR45 cause static encephalopathy of childhood with neurodegeneration in adulthood. Nat Genet. doi:10.1038/ng.2562
Yamanaka H, Gatanaga H, Kosalaraksa P, Matsuoka-Aizawa S, Takahashi T, Kimura S, Oka S (2007) Novel mutation of human DNA polymerase gamma associated with mitochondrial toxicity induced by anti-HIV treatment. J Infect Dis 195(10):1419–1425. doi:10.1086/513872
Honsho M, Tamura S, Shimozawa N, Suzuki Y, Kondo N, Fujiki Y (1998) Mutation in PEX16 is causal in the peroxisome-deficient Zellweger syndrome of complementation group D. Am J Hum Genet 63(6):1622–1630. doi:10.1086/302161
Grapp M, Just IA, Linnankivi T, Wolf P, Lucke T, Hausler M, Gartner J, Steinfeld R (2012) Molecular characterization of folate receptor 1 mutations delineates cerebral folate transport deficiency. Brain 135(Pt 7):2022–2031. doi:10.1093/brain/aws122
Steinfeld R, Grapp M, Kraetzner R, Dreha-Kulaczewski S, Helms G, Dechent P, Wevers R, Grosso S, Gartner J (2009) Folate receptor alpha defect causes cerebral folate transport deficiency: a treatable neurodegenerative disorder associated with disturbed myelin metabolism. Am J Hum Genet 85(3):354–363. doi:10.1016/j.ajhg.2009.08.005
Lukacs Z, Santavuori P, Keil A, Steinfeld R, Kohlschutter A (2003) Rapid and simple assay for the determination of tripeptidyl peptidase and palmitoyl protein thioesterase activities in dried blood spots. Clin Chem 49(3):509–511
Sohar I, Lin L, Lobel P (2000) Enzyme-based diagnosis of classical late infantile neuronal ceroid lipofuscinosis: comparison of tripeptidyl peptidase I and pepstatin-insensitive protease assays. Clin Chem 46(7):1005–1008
Ebberink MS, Csanyi B, Chong WK, Denis S, Sharp P, Mooijer PA, Dekker CJ, Spooner C, Ngu LH, De Sousa C, Wanders RJ, Fietz MJ, Clayton PT, Waterham HR, Ferdinandusse S (2010) Identification of an unusual variant peroxisome biogenesis disorder caused by mutations in the PEX16 gene. J Med Genet 47(9):608–615. doi:10.1136/jmg.2009.074302
Shimozawa N, Nagase T, Takemoto Y, Suzuki Y, Fujiki Y, Wanders RJ, Kondo N (2002) A novel aberrant splicing mutation of the PEX16 gene in two patients with Zellweger syndrome. Biochem Biophys Res Commun 292(1):109–112
Steinberg SJ, Dodt G, Raymond GV, Braverman NE, Moser AB, Moser HW (2006) Peroxisome biogenesis disorders. Biochim Biophys Acta 1763(12):1733–1748. doi:10.1016/j.bbamcr.2006.09.010
Kousi M, Lehesjoki AE, Mole SE (2012) Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinoses. Hum Mutat 33(1):42–63. doi:10.1002/humu.21624
Sun Y, Almomani R, Breedveld GJ, Santen GW, Aten E, Lefeber DJ, Hoff JI, Brusse E, Verheijen FW, Verdijk RM, Kriek M, Oostra B, Breuning MH, Losekoot M, den Dunnen JT, van de Warrenburg BP, Maat-Kievit AJ (2013) Autosomal recessive spinocerebellar ataxia 7 (SCAR7) is caused by variants in TPP1, the gene involved in classic late-infantile neuronal ceroid lipofuscinosis 2 disease (CLN2 disease). Hum Mutat 34(5):706–713. doi:10.1002/humu.22292
Haan J, Terwindt GM, van den Maagdenberg AM, Stam AH, Ferrari MD (2008) A review of the genetic relation between migraine and epilepsy. Cephalalgia 28(2):105–113. doi:10.1111/j.1468-2982.2007.01460.x
Roach JC, Glusman G, Smit AF, Huff CD, Hubley R, Shannon PT, Rowen L, Pant KP, Goodman N, Bamshad M, Shendure J, Drmanac R, Jorde LB, Hood L, Galas DJ (2010) Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science 328(5978):636–639. doi:10.1126/science.1186802
Lynch M (2010) Rate, molecular spectrum, and consequences of human mutation. Proc Natl Acad Sci U S A 107(3):961–968. doi:10.1073/pnas.0912629107
Durr A (2010) Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol 9(9):885–894. doi:10.1016/s1474-4422(10)70183-6
Huang L, Chardon JW, Carter MT, Friend KL, Dudding TE, Schwartzentruber J, Zou R, Schofield PW, Douglas S, Bulman DE, Boycott KM (2012) Missense mutations in ITPR1 cause autosomal dominant congenital nonprogressive spinocerebellar ataxia. Orphanet J Rare Dis 7:67. doi:10.1186/1750-1172-7-67
Srour M, Schwartzentruber J, Hamdan FF, Ospina LH, Patry L, Labuda D, Massicotte C, Dobrzeniecka S, Capo-Chichi JM, Papillon-Cavanagh S, Samuels ME, Boycott KM, Shevell MI, Laframboise R, Desilets V, Maranda B, Rouleau GA, Majewski J, Michaud JL (2012) Mutations in C5ORF42 cause Joubert syndrome in the French Canadian population. Am J Hum Genet 90(4):693–700. doi:10.1016/j.ajhg.2012.02.011
Parisi MA, Doherty D, Chance PF, Glass IA (2007) Joubert syndrome (and related disorders) (OMIM 213300). Eur J Hum Genet 15(5):511–521. doi:10.1038/sj.ejhg.5201648
Acknowledgments
We would like to thank all patients and their families for their participation in this study. We also thank Aya Narita and Nobuko Watanabe for technical assistance. This work was supported by the Ministry of Health, Labour, and Welfare of Japan; the Japan Society for the Promotion of Science (a Grant-in-Aid for Scientific Research (B) from (25293085, 25293235), a Grant-in-Aid for Scientific Research (A) (13313587)); the Takeda Science Foundation; the Japan Science and Technology Agency; the Strategic Research Program for Brain Sciences (11105137); and a Grant-in-Aid for Scientific Research on Innovative Areas (Transcription Cycle) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (12024421).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 283 kb)
Rights and permissions
About this article
Cite this article
Ohba, C., Osaka, H., Iai, M. et al. Diagnostic utility of whole exome sequencing in patients showing cerebellar and/or vermis atrophy in childhood. Neurogenetics 14, 225–232 (2013). https://doi.org/10.1007/s10048-013-0375-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-013-0375-8