Abstract
We describe the rare experience of veno–veno–arterial (VVA) extracorporeal membrane oxygenation (ECMO) in a patient with acute respiratory distress syndrome and septic-induced cardiomyopathy due to pulmonary tuberculosis (TB). A 24-year-old male patient who developed septic-induced cardiomyopathy secondary to pulmonary TB was administered veno-arterial (VA) ECMO for cardiac support. Six days later, the ECMO configuration mode was changed from VA to VVA to improve hypoxemia of the upper body and to prevent further lung injury. The patient was then successfully managed using an appropriate alternative ECMO strategy.
Similar content being viewed by others
Introduction
Pulmonary tuberculosis (TB) is a rare primary cause of acute respiratory distress syndrome (ARDS), and pulmonary TB patients requiring intensive care unit (ICU) admission and mechanical ventilator (MV) support have mortality rates ranging from 33 to 67% [1, 2]. Although severe active pulmonary TB is a disease which is controlled slowly, pulmonary function can deteriorate rapidly. This acute disease may be fulminant and include multiorgan failure and septic shock [3, 4]. Recent reports have demonstrated pulmonary TB patients with ARDS may be successfully treated by veno–venous (VV) extracorporeal membrane oxygenation (ECMO) [5, 6].
We report the case of a patient with ARDS and septic-induced cardiomyopathy caused by pulmonary TB who was successfully treated using an appropriate alternative ECMO configuration strategy.
Case report
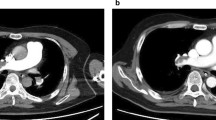
A 24-year-old man was admitted to our emergency room because of dyspnea. He had experienced weight loss (body weight 48 kg, height 173 cm) and a cough over the previous month ago but had not been evaluated or treated. At admission, his blood pressure (BP) was 125/75 mmHg, pulse 150 beats/min, respiratory rate 36 breaths/min, and temperature 36.8 °C. Arterial blood gas analysis (ABGA) showed PaO2 56.29 mmHg, PaCO2 29 mmHg, and SaO2 91%. Despite appropriate O2 supplementation, the desaturation persisted, and as a result mechanical ventilator (MV) care was initiated with an FiO2 of 0.6. A chest X-ray and computed tomography (CT) showed extensive bilateral pulmonary infiltrations (Fig. 1), and acid-fast staining of sputum was strongly smear-positive. Anti-TB drugs, including isoniazid (H) 300 mg, rifampicin (R) 600 mg, ethambutol (E) 800 mg and pyrazinamide (Z) 1500 mg, were administered through a nasogastric tube. A few days later, we confirmed the Mycobacterium tuberculosis with a sensitivity of H and R through acid-fast bacillus culture and TB polymerase chain reaction. Three days after admission, fever (39.2 °C) was observed and laboratory testing showed that white blood cells (WBC) and c-reactive protein (CRP) were elevated (WBC 10,670/mm3, CRP 17.09 mg/dl), but cardiac-specific enzymes were within normal limits. Despite high doses of inotropics and a vasopressor, systolic BP was below 80 mmHg (dobutamine 17.5 ug/kg/min, norepinephrine 0.04 ug/kg/min, vasopressin 0.5 iu/h). Despite appropriate volume replacement, norepinephrine and vasopressin have been increased to maintain a blood pressure. We considered clinically septic shock despite there was a diagnostic lack, such as blood culture. Pseudomonas aeruginosa was identified by sputum culture when the fever was generated. Transthoracic echocardiography (TTE) revealed depressed left ventricular (LV) systolic function and severe global hypokinesia (LV ejection fraction 12%). The ventilator setting was pressure control mode (PCV) with FiO2 0.45, positive end-expiratory pressure (PEEP) 7 cm H2O, inspiratory pressure level (IPL) 18 cm H2O and respiration rate (RR) 20/min. PaO2 69.5 mmHg, PCO2 34.1 mmHg and SaO2 94% were identified by ABGA. We considered that he developed septic-induced cardiomyopathy, and decided to apply veno–arterial (VA) ECMO on hospital day (HD) 4. Considering his respiratory function has been rapidly deteriorated due to pulmonary TB and combined pneumonia, we thought that VA ECMO was better than IABP because ECMO can help him to recover his respiratory function through the low ventilator settings. The dose of TB medications was not changed during the VA ECMO care. However, HRZ was quitted and a streptomycin 1 g was parenterally administrated on HD 7 because TB drug-induced hepatitis was identified (aspartate aminotransferase 116 U/L, alanine transaminase 59 U/L). H 300 mg, R 600 mg and Z 1500 mg were added through a nasogastric tube step by step. The streptomycin was quitted on HD 21 (Fig. 2).
Chest X-ray and CT images. (a) Serial chest radiographs demonstrating gradual resolution of lung infiltrates during ECMO support. (b) Initial chest CT revealed multifocal patchy consolidations in whole lung fields. After ECMO weaning, follow-up CT showed resolution of diffuse consolidation in middle and lower lungs. CT computed tomography, ECMO extracorporeal membrane oxygenation, VA veno–arterial, VVA veno–veno–arterial, VV veno–venous
Patient’s clinical course during ECMO. Table shows the ECMO setting and clinical data. AR aortic regurgitation, BP blood pressure, Dobu dobutamine, E ethambutol, ECMO extracorporeal membrane oxygenation, FiO 2 fraction of inspired oxygen, H isoniazid, HR heart rate, ICU intensive care unit, IPL inspiratory pressure level, LPM liter per minute, LVEF left ventricle ejection fraction, LVEDD left ventricle end diastolic dimension, LVESD left ventricle end systolic dimension, Norepi norepinephrine, MR mitral regurgitation, MV mechanical ventilator, PC pressure control, PEEP positive end-expiratory pressure, PS pressure support, R rifampin, RR respiration rate, RPM revolutions per minute, Strept. streptomycin, TTE trans thoracic echocardiography, TR tricuspid regurgitation, Vaso vasopressin, Z pyrazinamide
VA ECMO was applied via the right femoral artery and vein using a RotaFlow centrifugal pump (Maquet Cardiopulmonary AG, Hirrlingen, Germany), a 15-Frfemoral arterial cannula (Bio-Medicus, Medtronic Inc., Minneapolis, MN, USA), and a 24-Fr femoral venous cannula (RMI, Edward Life science, LLC, Irvine, CA, USA). His body surface area was calculated to be 1.56. The starting setting of VA EVMO was that flow was 3700 L/min (3800 revolutions/min), FiO2 was 0.8 and sweep gas flow was 0.35 L/min. A 16G catheter was inserted at right femoral artery for distal perfusion. After applying VA ECMO, inotropics and vasopressor were stopped and lactate declined from 5.3 mmol/L to the normal range.
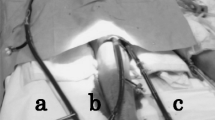
After 6 days on VA ECMO (on HD 10), cardiac function had improved, but serial right radial artery blood gas analyses demonstrated that hypoxemia was aggravated despite appropriate VA ECMO and MV setting. Despite ventilator setting was increased (PCV, FiO2 1.0, PEEP 6 cm H2O, IPL 20 cm H2O, RR 35/min), ABGA showed PaO2 58.1 mmHg, PaCO2 50.6 mmHg, and SaO2 86.5%. Upper body ischemia was suspected and the decision was made to change the ECMO configuration mode from VA to VVA for supporting an additional pulmonary support. Follow-up TTE (on HD 10) showed that cardiac function had not recovered completely (EF 17%). A 15-Fr return cannula was inserted in the right internal jugular vein. In addition, different circuit tube sizes were used for the arterial and venous return cannulas to control an excessive flow rate of venous return (arterial return 3/8′′, venous return 1/4′′). After instituting VVA ECMO, MV settings were minimized and upper body saturation improved. To prepare for protracted MV, tracheostomy was performed.
On HD 19 (after 9 days on VVA ECMO), cardiac function was sufficiently recovered to maintain hemodynamic stability without mechanical support or inotropics. However, respiratory function had not recovered due to the development of right pneumothorax on HD 15. Accordingly, VVA ECMO was converted to VV ECMO by removing an arterial return cannula. It was considered MV pressure-induced alveolar damage had aggravated the pneumothorax, and we decided on a MV wean while maintaining VV ECMO. An SaO2 of 92–96% at O2 4 L/min of T-piece was maintained under VV ECMO, which was finally discontinued on HD 28 (after 24 days of ECMO). The patient was transferred to a general ward on HD 39 and on HD 44 acid-fast staining was negative. During ECMO, bleeding at the tracheostomy site and closed thoracostomy site was occurred due to anticoagulation with heparin but we could control a bleeding with transfusion and adjusting a heparin dose. There were no other complications.
Discussion
Active pulmonary TB is a rare primary cause of acute respiratory failure, but high mortality rates have been recently reported in patients requiring ICU care due to ARDS arising from pulmonary TB [1, 2]. Furthermore, several reports have been issued on septic shock and cardiomyopathy from severe active disseminated TB [3, 4]. ECMO is a treatment option for ARDS or septic shock arising from pulmonary TB. In fact, several patients with ARDS from pulmonary TB were recently reported to be successfully rescued by ECMO [5,6,7,8,9]. Here we report for the first time the successful treatment of a patient with ARDS and septic-induced cardiomyopathy due to pulmonary TB by VVA ECMO.
Early anti-TB medication is critical in severe active pulmonary TB, and appropriate mechanical support, such as MV and ECMO, might improve the survivals of patients with systemic organ failure. Our patient was rescued with the early anti-TB medication and ECMO.
ECMO is usually employed in either the VV or VA modes, and several studies on ARDS have shown that VV ECMO reduces mortality [10,11,12]. Studies on VVA ECMO in ARDS are limited. In a small-cohort study, it was concluded that VVA ECMO appears to improve survival in ARDS [13], and Lus et al. reported that VVA ECMO provides a technically feasible rescue strategy for the treatment of combined respiratory and hemodynamic failure [14]. The configuration mode used for ECMO should be decided upon based on the support required. If cardiac and respiratory failure co-exist, blood return to both venous and arterial systems, that is VVA ECMO configuration, may be instituted. In the described case, the configuration mode should be changed at an appropriate time based on continuous monitoring and echocardiography data, and then the patient was successfully weaned off ECMO without complications. VVA ECMO does not necessarily apply to all patients which was needed cardiac and respiratory support. If the ventilator can support the respiratory function and the upper body hypoxia does not progress, VVA ECMO does not need to apply. However, we think that VVA mode will be better in patients with ARDS who are expected to rapidly recover by lowering the ventilator setting as in our patient.
In the VVA ECMO configuration, flow through the two return cannulas should be balanced using separate flow sensors and an adjustable clamp. In our patient, we controlled flows using different size of circuit tubes because at the time we did not have either of these devices to measure and adjust the flow. Usually, the resistance of the superior vena cava is lower than that of femoral artery and, thus, if the tube sizes of the two return cannulas are the same, venous return exceeds arterial return. Accordingly, we used a smaller tube on the venous return, and monitored hemodynamic status and radial ABGA to confirm that the flow was appropriate. The objective parameters are blood pressure, results of ABGA, mixed venous SO2 (SvO2) and trends of lactate. Especially, PaO2 of the right radial artery is the indicator to identify upper body hypoxia. If the flow needed to adjust, we would plan to clamp the tube, partially.
In our patient, secondary pneumothorax occurred presumably due to pulmonary TB and MV. Positive pressure of mechanical ventilation can aggravate pneumothorax [15]. As a result, we weaned the patient off MV and retained VV ECMO.
We describe our primitive experience of a 24-year-old man with ARDS and septic-induced cardiomyopathy secondary to severe active pulmonary TB, who was rescued by VVA ECMO. We suggest that VVA ECMO is considered an alternative strategy to assist heart and lungs in patients with combined cardiopulmonary failure secondary to severe active pulmonary TB.
Conclusions
Severe active pulmonary TB can cause cardiopulmonary function to deteriorate rapidly, and VVA ECMO may be an effective rescue modality in affected patients. Furthermore, we recommend appropriate changes in ECMO configuration be made based on considerations of cardiopulmonary function to minimize complications.
References
Hagan G, Nathani N. Clinical review: tuberculosis on the intensive care unit. Crit Care. 2013;17:240.
Silva DR, Gazzana MB, DalcinPde T. Severe tuberculosis requiring ICU admission. J Bras Pneumol. 2012;38:386–94.
Sydow M, Schauer A, Crozier TA, Burchardi H. Multiple organ failure in generalized disseminated tuberculosis. Respir Med. 1992;86:517–9.
Ahuja SS, Ahuja SK, Phelps KR, Thelmo W, Hill AR. Hemodynamic confirmation of septic shock in disseminated tuberculosis. Crit Care Med. 1992;20:901–3.
Nam SJ, Cho YJ. The successful treatment of refractory respiratory failure due to miliary tuberculosis: survival after prolonged extracorporeal membrane oxygenation support. Clin Respir J. 2016;10:393–9.
Omote N, Kondoh Y, Taniguchi H, Kimura T, Kataoka K, Hasegawa R, et al. Acute respiratory distress syndrome due to severe pulmonary tuberculosis treated with extracorporeal membrane oxygenation: a case report and review of the literature. Respir Med Case Rep. 2016;10:31–3.
Homan W, Harman E, Braun NM, Felton CP, King TK, Smith JP. Miliary tuberculosis presenting as acute respiratory failure: treatment by membrane oxygenator and ventricle pump. Chest. 1975;67:366–9.
Petrillo TM, Heard ML, Fortenberry JD, Stockwell JA, Leonard MK Jr. Respiratory failure caused by tuberculous pneumonia requiring extracorporeal membrane oxygenation. Perfusion. 2001;16:525–9.
Mauri T, Foti G, Zanella A, Bombino M, Confalonieri A, Patroniti N, et al. Long-term extracorporeal membrane oxygenation with minimal ventilatory support: a new paradigm for severe ARDS? Minerva Anestesiol. 2012;78:385–9.
MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med. 2012;38:210–20.
Combes A, Bacchetta M, Brodie D, Müller T, Pellegrino V. Extracorporeal membrane oxygenation for respiratory failure in adults. Curr Opin Crit Care. 2012;18:99–104.
Seo DJ, Yoo JS, Kim JB, Jung SH, Choo SJ, Chung CH, et al. Venovenous extracorporeal membrane oxygenation for postoperative acute respiratory distress syndrome. Korean J Thorac Cardiovasc Surg. 2015;48:180–6.
Stöhr F, Emmert MY, Lachat ML, Stocker R, Maggiorini M, Falk V, et al. Extracorporeal membrane oxygenation for acute respiratory distress syndrome: is the configuration mode an important predictor for the outcome? Interact Cardiovasc Thorac Surg. 2011;12:676–80.
Ius F, Sommer W, Tudorache I, Avsar M, Siemeni T, Salman J, et al. Veno–veno–arterial extracorporeal membrane oxygenation for respiratory failure with severe haemodynamic impairment: technique and early outcomes. Interact Cardiovasc Thorac Surg. 2015;20:761–7.
Hsu C-W, Sun S-F. Iatrogenic pneumothorax related to mechanical ventilation. World J Crit Care Med. 2014;3:8–14.
Acknowledgments
The authors declare that funding was not utilized for the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to report.
Rights and permissions
About this article
Cite this article
Lee, S.I., Hwang, H.J., Lee, S.Y. et al. Veno–veno–arterial extracorporeal membrane oxygenation for acute respiratory distress syndrome with septic-induced cardiomyopathy due to severe pulmonary tuberculosis. J Artif Organs 20, 359–364 (2017). https://doi.org/10.1007/s10047-017-0982-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-017-0982-5