Abstract
Purpose
We conducted a network meta-analysis to evaluate potential differences in patient outcomes when different meshes, especially biological meshes, were used for ventral hernia repair.
Methods
PubMed, Embase, Cochrane Library, and Clinical Trials.gov databases were searched for studies comparing biological meshes with biological or synthetic meshes for ventral hernia repair. The outcomes were hernia recurrence rate, surgical site infection, and seroma. We performed a two-step network meta-analysis to investigate the outcomes of several biological meshes: non-cross-linked human acellular dermal matrix (NCHADM), non-cross-linked porcine ADM (NCPADM), non-cross-linked bovine ADM (NCBADM), cross-linked porcine ADM (CPADM), and porcine small intestinal submucosa (PSIS).
Results
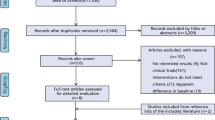
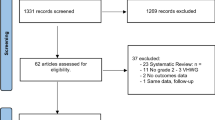
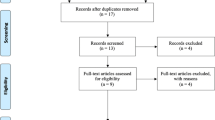
From 6304 publications, 23 studies involving 2603 patients were finally included. We found no differences between meshes in recurrence at 1-year follow-up and in surgical site infection rate. NCBADM was associated with the lowest recurrence rate and the lowest surgical site infection rate. NCHADM implantation was associated with the lowest rate of seroma. PSIS was associated with a higher risk of seroma than NCHADM (pooled risk ratio 3.89, 95% confidence interval 1.13–13.39) and NCPADM (RR 3.42, 95% CI 1.29–9.06).
Conclusions
Our network meta-analysis found no differences in recurrence rate or surgical site infection among different biological meshes. The incidence of postoperative seroma was higher with PSIS than with acellular dermal matrices. We observed large heterogeneity in the studies of ventral hernia repair using biological meshes, and, therefore, well-designed randomized clinical trials are needed.



Similar content being viewed by others
References
Rosen MJ, Bauer JJ, Harmaty M et al (2017) Multicenter, prospective, longitudinal study of the recurrence, surgical site infection, and quality of life after contaminated ventral hernia repair using biosynthetic absorbable mesh: the COBRA study. Ann Surg 265:205–211. https://doi.org/10.1097/SLA.0000000000001601
Lazzati A, Nassif GB, Paolino L (2018) Concomitant ventral hernia repair and bariatric surgery: a systematic review. Obes Surg 28:2949–2955. https://doi.org/10.1007/s11695-018-3366-x
Yang S, Chen J, Shen Y-M et al (2018) Retrospective research on initiative content reduction technique for obesity patients with huge abdominal incisional hernia. Int J Abdom Wall Hernia Surg 1:19. https://doi.org/10.4103/ijawhs.ijawhs_2_18
Luijendijk RW, Hop WCJ, van den Tol MP et al (2000) A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 343:392–398. https://doi.org/10.1056/NEJM200008103430603
Parker SG, Mallett S, Quinn L et al (2021) Identifying predictors of ventral hernia recurrence: systematic review and meta-analysis. BJS Open 5:zraa071. https://doi.org/10.1093/bjsopen/zraa071
Cobb WS (2018) A current review of synthetic meshes in abdominal wall reconstruction. Plast Reconstr Surg 142:64S-71S. https://doi.org/10.1097/PRS.0000000000004857
Morris MP, Mellia JA, Christopher AN et al (2021) Ventral hernia repair with synthetic mesh in a contaminated field: a systematic review and meta-analysis. Hernia 25:1035–1050. https://doi.org/10.1007/s10029-020-02358-5
Rosen MJ, Krpata DM, Ermlich B, Blatnik JA (2013) A 5-year clinical experience with single-staged repairs of infected and contaminated abdominal wall defects utilizing biologic mesh. Ann Surg 257:991–996. https://doi.org/10.1097/SLA.0b013e3182849871
Warren J, Desai SS, Boswell ND et al (2020) Safety and efficacy of synthetic mesh for ventral hernia repair in a contaminated field. J Am Coll Surg 230:405–413. https://doi.org/10.1016/j.jamcollsurg.2019.12.008
Shao JM, Ayuso SA, Deerenberg EB et al (2021) Biologic mesh is non-inferior to synthetic mesh in CDC class 1 & 2 open abdominal wall reconstruction. Am J Surg. https://doi.org/10.1016/j.amjsurg.2021.05.019
Miserez M, Lefering R, Famiglietti F et al (2021) Synthetic versus biological mesh in laparoscopic and open ventral hernia repair (LAPSIS): results of a multinational, randomized, controlled, and double-blind trial. Ann Surg 273:57–65. https://doi.org/10.1097/sla.0000000000004062
Maxwell DW, Hart AM, Keifer OP et al (2019) A comparison of acellular dermal matrices in abdominal wall reconstruction. Ann Plast Surg 82:435–440. https://doi.org/10.1097/sap.0000000000001692
Gupta A, Zahriya K, Mullens PL et al (2006) Ventral herniorrhaphy: experience with two different biosynthetic mesh materials, surgisis and alloderm. Hernia 10:419–425. https://doi.org/10.1007/s10029-006-0130-2
Sterne JA, Hernán MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ. https://doi.org/10.1136/bmj.i4919
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Taibi A, Derbal S, Durand Fontanier S et al (2021) Implantation of biologic mesh in ventral hernia repair—does it make sense? Surg Endosc 35:702–709. https://doi.org/10.1007/s00464-020-07435-5
Harris HW, Primus F, Young C et al (2021) Preventing recurrence in clean and contaminated hernias using biologic versus synthetic mesh in ventral hernia repair: the PRICE randomized clinical trial. Ann Surg 273:648–655. https://doi.org/10.1097/sla.0000000000004336
Charleux-Muller D, Hurel R, Fabacher T et al (2021) Slowly absorbable mesh in contaminated incisional hernia repair: results of a French multicenter study. Hernia 25:1051–1059. https://doi.org/10.1007/s10029-020-02366-5
Carbonell AM, Warren JA, Prabhu AS et al (2018) Reducing length of stay using a robotic-assisted approach for retro muscular ventral hernia repair: a comparative analysis from the Americas hernia society quality collaborative. Ann Surg 267:210–217. https://doi.org/10.1097/SLA.0000000000002244
Novitsky YW, Rosen MJ (2012) The biology of biologics. Plast Reconstr Surg 130:9S-17S. https://doi.org/10.1097/PRS.0b013e31825f395b
Trippoli S, Caccese E, Tulli G et al (2018) Biological meshes for abdominal hernia: lack of evidence-based recommendations for clinical use. Int J Surg 52:278–284. https://doi.org/10.1016/j.ijsu.2018.02.046
Samson DJ, Gachabayov M, Latifi R (2021) Biologic mesh in surgery: a comprehensive review and meta-analysis of selected outcomes in 51 studies and 6079 patients. World J Surg 45:3524–3540. https://doi.org/10.1007/s00268-020-05887-3
Buell JF, Sigmon D, Ducoin C et al (2017) Initial experience with biologic polymer scaffold (poly-4-hydroxybuturate) in complex abdominal wall reconstruction. Ann Surg 266:185–188. https://doi.org/10.1097/SLA.0000000000001916
Itani KMF, Rosen M, Vargo D et al (2012) Prospective study of single-stage repair of contaminated hernias using a biologic porcine tissue matrix: the RICH Study. Surgery 152:498–505. https://doi.org/10.1016/j.surg.2012.04.008
Smart NJ, Marshall M, Daniels IR (2012) Biological meshes: a review of their use in abdominal wall hernia repairs. The Surgeon 10:159–171. https://doi.org/10.1016/j.surge.2012.02.006
Cao G, Wang C, Fan Y, Li X (2020) Biomimetic SIS-based bio composites with improved biodegradability, antibacterial activity and angiogenesis for abdominal wall repair. Mater Sci Eng: C 109:110538. https://doi.org/10.1016/j.msec.2019.110538
Rose JF, Zafar SN, Ellsworth Iv WA (2016) Does acellular dermal matrix thickness affect complication rate in tissue expander based breast reconstruction? Plast Surg Int 2016:2867097. https://doi.org/10.1155/2016/2867097
Cheng W, Chen J, Liu Y et al (2019) Experimental assessment of tissue repair of basement membrane in partial thickness defect in abdominal wall of rats. Chin J Hernia Abdom Wall Surg (Electron Ed) 13:198–203
Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, Badylak SF (2007) Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am 89:621–630. https://doi.org/10.2106/JBJS.E.00742
Jiang W, Zhang J, Lv X et al (2016) Use of small intestinal submucosal and acellular dermal matrix grafts in giant omphaloceles in neonates and a rabbit abdominal wall defect model. J Pediatr Surg 51:368–373. https://doi.org/10.1016/j.jpedsurg.2015.08.005
Record RD, Hillegonds D, Simmons C et al (2001) In vivo degradation of 14C-labeled small intestinal submucosa (SIS) when used for urinary bladder repair. Biomaterials 22:2653–2659. https://doi.org/10.1016/s0142-9612(01)00007-2
Cao G, Huang Y, Li K et al (2019) Small intestinal submucosa: superiority, limitations and solutions, and its potential to address bottlenecks in tissue repair. J Mater Chem B 7:5038–5055. https://doi.org/10.1039/C9TB00530G
Nessel R, Löffler T, Rinn J et al (2021) Primary and recurrent repair of incisional hernia based on biomechanical considerations to avoid mesh-related complications. Front Surg 8:764470. https://doi.org/10.3389/fsurg.2021.764470
Todros S, Pavan PG, Pachera P, Natali AN (2017) Synthetic surgical meshes used in abdominal wall surgery: part II-biomechanical aspects. J Biomed Mater Res B Appl Biomater 105:892–903. https://doi.org/10.1002/jbm.b.33584
Tomaszewska A, Lubowiecka I, Szymczak C (2019) Mechanics of mesh implanted into abdominal wall under repetitive load. Experimental and numerical study. J Biomed Mater Res B Appl Biomater 107:1400–1409. https://doi.org/10.1002/jbm.b.34232
Kallinowski F, Baumann E, Harder F et al (2015) Dynamic intermittent strain can rapidly impair ventral hernia repair. J Biomech 48:4026–4036. https://doi.org/10.1016/j.jbiomech.2015.09.045
Tulloh B, de Beaux A (2016) Defects and donuts: the importance of the mesh:defect area ratio. Hernia 20:893–895. https://doi.org/10.1007/s10029-016-1524-4
Mulder IM, Deerenberg EB, Bemelman WA et al (2015) Infection susceptibility of crosslinked and non-crosslinked biological meshes in an experimental contaminated environment. Am J Surg 210:159–166. https://doi.org/10.1016/j.amjsurg.2014.06.025
Cheng AW, Abbas MA, Tejirian T (2014) Outcome of abdominal wall hernia repair with biologic mesh: PermacolTM versus StratticeTM. Am Surg 80:999–1002. https://doi.org/10.1177/000313481408001019
Shah BC, Tiwari MM, Goede MR et al (2011) Not all biologics are equal! Hernia 15:165–171. https://doi.org/10.1007/s10029-010-0768-7
Ban KA, Minei JP, Laronga C et al (2017) American college of surgeons and surgical infection society: surgical site infection guidelines, 2016 update. J Am Coll Surg 224:59–74. https://doi.org/10.1016/j.jamcollsurg.2016.10.029
Fan JKM, Yip J, Foo DCC et al (2017) Randomized trial comparing self-gripping semi re-absorbable mesh (PROGRIP) with polypropylene mesh in open inguinal hernioplasty: the 6 years result. Hernia 21:9–16. https://doi.org/10.1007/s10029-016-1545-z
Morales-Conde S (2012) A new classification for seroma after laparoscopic ventral hernia repair. Hernia 16:261–267. https://doi.org/10.1007/s10029-012-0911-8
Sweitzer K, Carruthers KH, Blume L et al (2021) The biomechanical properties of meshed versus perforated acellular dermal matrices (ADMs). Plast Reconstr Surg Glob Open 9:e3454. https://doi.org/10.1097/GOX.0000000000003454
Liu Y, Cao Z, Yang H et al (2020) Porcine small intestinal submucosa mesh to treat inguinal hernia in young adults using laparoscopic inguinal hernia repair: a retrospective controlled study. Surg Laparosc Endosc Percutan Tech 30:367–370. https://doi.org/10.1097/SLE.0000000000000806
Ravo B, Falasco G (2020) Pure tissue inguinal hernia repair with the use of biological mesh: a 10-year follows up. A prospective study. Hernia 24:121–126. https://doi.org/10.1007/s10029-019-01976-y
Ansaloni L, Cambrini P, Catena F et al (2007) Immune response to small intestinal submucosa (Surgisis) implant in humans: preliminary observations. J Invest Surg 20:237–241. https://doi.org/10.1080/08941930701481296
Daly KA, Stewart-Akers AM, Hara H et al (2009) Effect of the alphaGal epitope on the response to small intestinal submucosa extracellular matrix in a nonhuman primate model. Tissue Eng Part A 15:3877–3888. https://doi.org/10.1089/ten.TEA.2009.0089
Helton WS, Fisichella PM, Berger R et al (2005) Short-term outcomes with small intestinal submucosa for ventral abdominal hernia. Arch Surg 140:549–560. https://doi.org/10.1001/archsurg.140.6.549
Daly KA, Liu S, Agrawal V et al (2012) The host response to endotoxin-contaminated dermal matrix. Tissue Eng Part A 18:1293–1303. https://doi.org/10.1089/ten.TEA.2011.0597
Flum DR, Horvath K, Koepsell T (2003) Have outcomes of incisional hernia repair improved with time? A population-based analysis. Ann Surg 237:129–135. https://doi.org/10.1097/00000658-200301000-00018
Brescia A, Tomassini F, Berardi G et al (2016) Post-incisional ventral hernia repair in patients undergoing chemotherapy: improving outcomes with biological mesh. World J Surg Oncol 14:257. https://doi.org/10.1186/s12957-016-1011-5
Brewer MB, Rada EM, Milburn ML et al (2011) Human acellular dermal matrix for ventral hernia repair reduces morbidity in transplant patients. Hernia 15:141–145. https://doi.org/10.1007/s10029-010-0748-y
Byrge N, Mone MC, Vargo D (2017) Hospital wide porcine mesh conversion results in cost savings with equivalent clinical outcomes. Am J Surg 213:1042–1045. https://doi.org/10.1016/j.amjsurg.2017.01.027
Clemens MW, Selber JC, Liu J et al (2013) Bovine versus porcine acellular dermal matrix for complex abdominal wall reconstruction. Plast Reconstr Surg 131:71–79. https://doi.org/10.1097/PRS.0b013e3182729e58
Cobb GA, Shaffer J (2005) Cross-linked acellular porcine dermal collagen implant in laparoscopic ventral hernia repair: case-controlled study of operative variables and early complications. Int Surg 90:S24–S29
de Vries FEE, Hodgkinson JD, Claessen JJM et al (2020) Long-term outcomes after contaminated complex abdominal wall reconstruction. Hernia 24:459–468. https://doi.org/10.1007/s10029-020-02124-7
Fischer JP, Basta MN, Mirzabeigi MN, Kovach SJ (2014) A comparison of outcomes and cost in VHWG grade II hernias between Rives-Stoppa synthetic mesh hernia repair versus underlay biologic mesh repair. Hernia 18:781–789. https://doi.org/10.1007/s10029-014-1309-6
Iacco A, Adeyemo A, Riggs T, Janczyk R (2014) Single institutional experience using biological mesh for abdominal wall reconstruction. Am J Surg 208:480–484. https://doi.org/10.1016/j.amjsurg.2013.09.020
Janfaza M, Martin M, Skinner R (2012) A preliminary comparison study of two noncrosslinked biologic meshes used in complex ventral hernia repairs. World J Surg 36:1760–1764. https://doi.org/10.1007/s00268-012-1576-2
Ko JH, Wang EC, Salvay DM et al (2009) Abdominal wall reconstruction: lessons learned from 200 “components separation” procedures. Arch Surg 144:1047–1055. https://doi.org/10.1001/archsurg.2009.192
Koscielny A, Widenmayer S, May T et al (2018) Comparison of biological and alloplastic meshes in ventral incisional hernia repair. Langenbecks Arch Surg 403:255–263. https://doi.org/10.1007/s00423-017-1639-9
Liang MK, Berger RL, Nguyen MT et al (2014) Outcomes with porcine acellular dermal matrix versus synthetic mesh and suture in complicated open ventral hernia repair. Surg Infect (Larchmt) 15:506–512. https://doi.org/10.1089/sur.2013.090
Majumder A, Winder JS, Wen Y et al (2016) Comparative analysis of biologic versus synthetic mesh outcomes in contaminated hernia repairs. Surgery 160:828–838. https://doi.org/10.1016/j.surg.2016.04.041
Olavarria OA, Bernardi K, Dhanani NH et al (2021) Synthetic versus biologic mesh for complex open ventral hernia repair: a pilot randomized controlled trial. Surg Infect 22:496–503. https://doi.org/10.1089/sur.2020.166
Romain B, Story F, Meyer N et al (2016) Comparative study between biologic porcine dermal meshes: risk factors of postoperative morbidity and recurrence. J Wound Care 25:320–5. https://doi.org/10.12968/jowc.2016.25.6.320
Sailes FC, Walls J, Guelig D et al (2011) Ventral hernia repairs: 10-year single-institution review at Thomas Jefferson university hospital. J Am Coll Surg 212:119–123. https://doi.org/10.1016/j.jamcollsurg.2010.08.021
Funding
The study was funded by National Natural Science Foundation of China (NSFC) (31771043); National defense science and technology excellence youth science fund (2019-JCJQ-ZQ-002); National Defense Science and technology foundation strengthening plan (2019-JCJQ-JJ-069).
Author information
Authors and Affiliations
Contributions
HZ and ZZ did the literature search. JC and JZ formed the study design. Data collection done by HZ, XL and ZZ. HZ and XL analyzed the data. JZ and YS interpreted the data. HZ wrote the manuscript. YS, JZ and JC critically reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval is not considered, because it is a systematic review of the published literature.
Human and animal rights
This study does not include human or animal participants.
Informed consent
Informed consent was not required for this review study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, H., Shen, Y., Zhang, Z. et al. Comparison of outcomes of ventral hernia repair using different meshes: a systematic review and network meta-analysis. Hernia 26, 1561–1571 (2022). https://doi.org/10.1007/s10029-022-02652-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-022-02652-4