Abstract
Purpose
To evaluate if incisional prophylactic negative pressure wound therapy (pNPWT) reduces wound infections and other wound complications in high-risk patients undergoing major complex ventral abdominal wall repair.
Methods
Retrospective before–after comparison nested in a consecutive series of patients undergoing elective major complex abdominal wall repair. Starting January 2014, pNPWT was applied on the closed incisional wound for a minimum of 5 days. To minimize selection bias, we compared two periods of 14 months before and after January 2014. Wound infections according to the Centre for Disease Control Surgical Site Infection classification as well as other wound complications were recorded.
Results
Thirty-two patients were included in the pNPWT group and 34 in the control group. The study group involved clean-contaminated and contaminated operations due to enterocutaneous fistula, enterostomies or infected mesh. Median duration of pNPWT was 5 days (IQR 5–7). Overall wound infection rate was 35%. pNPWT was associated with a significant decrease in postoperative wound infection rate (24 versus 51%; p = 0.029, OR 0.30 (95% CI 0.10–0.90)). Incisional wound infection rates dropped from 48 to 7% (p < 0.01, OR 0.08 (95% CI 0.16–0.39), whereas the number of subcutaneous abscesses was comparable in both groups. Moreover, less interventions were needed in the pNPWT group (p < 0.001).
Conclusions
Closed incision pNPWT seems a promising solution to reduce the incidence of wound infections in complex abdominal wall surgery. Randomized controlled trials are needed to estimate more precisely the value and cost-effectiveness of pNPWT in this high-risk setting.
Similar content being viewed by others
Introduction
Patients undergoing major complex abdominal wall repair (CAWR) are at high risk to develop wound complications [1, 2]. Some of these operations are contaminated or even dirty, for example in patients with enterocutaneous fistulas (ECF), enterostomies or infected mesh. Furthermore, abdominal wall reconstruction may require component separation techniques (CST), sometimes combined with the use of a mesh and flap reconstructions. Increased wound surface and the implantation of a foreign body can, therefore, increase the risk of wound complications. Last but not least, a considerable amount of these patients have a history of repeated abdominal surgery, recurrent hernias, long-lasting hospital stay and dependence of parenteral nutrition (PN). Altogether, their physical and nutritional condition is most often suboptimal and these patients are prone to wound complications.
Reported rates of surgical site occurrences in this setting are 29–66% [3,4,5,6,7,8,9,10]. Surgical site infections (SSI) and other wound complications are associated with high morbidity, considerable mortality, longer hospital stay and increased health care costs [11]. Several patient characteristics such as smoking and obesity, but also operative characteristics such as length of operation and the level of contamination, are associated with an increased risk of developing SSI [11]. Over the last decades, several measures to reduce SSI have been implemented such as systemic antibiotic prophylaxis and antiseptic agents used to rinse the operative field [12, 13]. Since the introduction of negative pressure wound therapy (NPWT) in 1995, its popularity has grown and it has shown potential for several indications such as open bone fractures [14], diabetic ulcers [15] and the open abdomen [16]. First introduced in orthopaedic surgery in 2006 [17], prophylactic negative pressure wound therapy (pNPWT) has been suggested as a new method to prevent wound complications by its application on a closed incisional wound. NPWT consists of a closed, sealed system connected to a vacuum pump, which maintains negative pressure on the wound. The precise mechanism of pNPWT is unknown. Current hypothesis is that pNPWT creates a moist wound healing environment, drains exudate, reduces tissue edema, contracts the wound edges, mechanically stimulates the wound bed, and influences blood perfusion at the wound edge, which may lead to angiogenesis and the formation of granulation tissue [18]. Moreover, the sealed system might protect against micro-organisms from outside entering the wound.
Conflicting results have been reported on the use of pNPWT. The aim of the present study was to evaluate if pNPWT reduces the incidence of wound infections and other wound complications in patients undergoing elective open major CAWR.
Methods
Study design and data collection
This was a retrospective before–after comparison nested in a consecutive series of patients undergoing CAWR at the Department of Surgery of the Academic Medical Center in Amsterdam, a tertiary university hospital in the Netherlands. The medical ethical committee of the hospital approved the study and for this type of study, formal consent was not required. Starting January 2014, the use of pNPWT for patients undergoing complex abdominal wall repair became a standard part of the procedure. To minimize selection bias, we compared two periods of 14 months before and 14 months after January 2014 to assess the effects of pNPWT. All data on operations performed between September 2012 and April 2015 by a single surgeon, more than 10 years specialized in complex abdominal wall surgery and lead of a specialized outpatient clinic for acute intestinal failure, were reviewed. Primary outcome was the incidence of superficial and deep wound infections. The wound infections were further subdivided into incisional wound infections and/or subcutaneous abscesses. Secondary outcomes were the appearance of other wound complications such as non-infected seroma, bleeding or hematoma, skin- or fat necrosis, wound dehiscence requiring (prolonged) NPWT, anastomotic leakage, intra-abdominal abscesses, ECF, the need for percutaneous or surgical interventions, emergency department visits after discharge, re-admissions and 30-day mortality. All adult patients undergoing midline elective open, CAWR according to the criteria of major complex abdominal wall surgery as described by Slater in 2014 [2] were included. Primary skin closure at the end of surgery (with or without flap rotation by a plastic surgeon) was a prerequisite. Data on patient demographics, risk factors for SSI, level of contamination, operative characteristics and post-operative complications (including SSI) were extracted from the clinical charts.
Outcome and definitions
Infections were scored from the clinical charts after the operative procedure and classified according to the Centre for Disease Control (CDC) SSI criteria (including superficial, deep and organ space infections). Next to this, superficial wound infections according to the CDC SSI criteria were scored for subcutaneous abscess and/or incisional wound infection. In case a culture was available these results were used, if no culture was available information from the clinical chart was used. The description of purulent drainage from a superficial incision or the need of opening the wound by the surgeon due to pain or tenderness, localized swelling, erythema or heat was scored as positive for wound infection. In case of bowel content leakage from either the anastomosis or bowel perforation which was proven by contrast radiography or relaparotomy, this was scored as leakage. If there was an unnatural communication between the gastro-intestinal tract and the skin, it was registered as an ECF. Intra-abdominal fluid collections with positive culture after drainage without signs of anastomotic leakage or bowel perforation were scored as an intra-abdominal abscess. Wound dehiscence needing postoperative therapeutic NPWT was recorded separately in the control group as well as in the pNPWT group. For the pNPWT group, this element was scored if NPWT was continued, after the first dressing change at postoperative day 5, because of dehiscence during pNPWT. Continuing pNPWT at the dressing change at postoperative day 5 because of severe edema but without any signs of dehiscence or infection was not scored as SSI. Bleeding or hematoma was recorded in the case of either active bleeding or a large hematoma, which needed radiologic drainage. We defined seroma as a subcutaneous fluid collection without any signs of infection or with negative culture results after drainage. Infected seroma was scored as a superficial SSI. Interventions were scored separately for intra-abdominal and extra-abdominal. Intra-abdominal interventions could be either radiologic percutaneous drainage or relaparotomy. Extra-abdominal interventions were scored for radiologic percutaneous subcutaneous drainage and the need for opening of the wound with or without antibiotics. Opening of the wound could be either spontaneously due to infection or as part of therapeutic drainage. Interventions were scored for infectious complications as well as non-infectious complications.
Surgical technique
Operative principles for CAWR did not change over the study period apart from the application of pNPWT. Infection prevention was performed according to the national guidelines and included hand hygiene, surgical site preparation with an alcohol and chlorhexidine based, non-aqueous, antiseptic agent and systemic prophylactic antibiotics 30–60 min before incision. In case of presence of ECF segmental intestinal resection was performed. All patients had hand-sewn anastomoses. In patients where primary fascial closure was not possible, an open anterior CST was performed. An intra-abdominal sublay biological mesh (Strattice™ porcine dermal matrix, Acelity) was used in all cases with enterocutaneous or enteroatmospheric fistula and sometimes in other contaminated situations, such as infected synthetic mesh removal. In clean situations, a synthetic retrorectus light-weight polypropylene sublay Vypro® mesh (Ethicon/Johnson&Johnson) was used. Sometimes, in very large clean abdominal wall defects a double layer technique was used, including intra-abdominal sublay Strattice ™ dermal matrix and retrorectus light-weight polypropylene sublay Vypro® mesh, combined with a CST. On average, three 18 Fr suction drains were used—1 subfascial on top of the biological mesh and 2 subcutaneous—to ensure active drainage of fluids and these were only removed when production was <30 ml/24 h. The fascia was closed with a monofilament polydioxanone suture (PDS loop). In case of large full thickness skin defects, a plastic surgeon performed a flap reconstruction. No skin grafts were used. All patients had subcutical closure before the skin was closed. The skin was closed with single polyester Mersilene® sutures (Ethicon); no staples were used. Up to January 2014, elastic Microfoam surgical bandage (3 M) was applied for 24 h. From January 2014, pNPWT (either Prevena™ (Acelity/KCI) or a home-made construction of Melolin non-adhesive wound dressing (Smith&Nephew) and V.A.C® Granufoam™ (Acelity/KCI) was used for at least 5 days with a negative pressure of 100 mmHg. After 5 days, the surgical team performed wound inspection. In case of severe edema and/or risk of wound dehiscence due to abdominal distension, pNPWT dressing was changed and prolonged for another 3–5 days. In case of a closed wound without any signs of edema or dehiscence, the pNPWT was removed. All patients wore a binder for 7 days continuously and thereafter for 3 months postoperative when mobilizing.
Statistical analyses
The pNPWT and the control group were compared using Chi-square (Fisher exact) test for categorical data and using Mann–Whitney U test for not normal distributed numerical variables. Normal continuous numerical variables were compared using Students t test only if found normally distributed in a Q–Q plot. Statistical analyses were performed with SPSS version 21.0. A p value ≤ 0.05 was considered statistically significant.
Results
Study population and operative characteristics
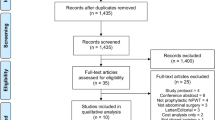
During the 14-month period, from January 2014 until April 2015, 32 consecutive patients undergoing CAWR fulfilled the inclusion criteria and were treated with pNPWT. In the preceding 14 months, between September 2012 and January 1st 2014, a consecutive group of 34 patients formed the control group. Of these 66 consecutive patients, 36 (55%) were male, and the mean age at the time of operation was 59.4 [standard deviation (SD) 13.3] years. Baseline characteristics regarding co-morbidity known for increased risk of SSI, such as obesity or inflammatory bowel disease (IBD), did not significantly differ between both groups (Table 1). Overall, almost half of the included patients (44%) depended on PN. Seventy-seven percent (51/66) of the operations involved clean-contaminated or contaminated or dirty operations. The median number of previous abdominal surgeries was 4 (range 1–24). In 91% of the patients, a CST was performed, usually on both sides. Mean operation time (including anesthesia) was 438 (SD 153) minutes. Surgery was performed for the following reasons: resection of one or more enteric fistulas, restorations of bowel continuity, very large or recurrent hernias or recurrent hernia with infected mesh. Reconstruction by a plastic surgeon was required in 12 cases (18%). In Table 1, we summarize patient and operative characteristics for both the pNPWT and the control group. There were no statistical significant differences in operative characteristics between the two groups.
Outcome
Table 2 summarizes outcomes of both groups. Overall SSI rate (including organ-space infections) was 47%. Six patients developed anastomotic leakage. In these patients, it was not possible to judge SSI; thus, these six patients were excluded from the wound infection analysis. The wound infection rate was 38% (23/66). We had no deep SSI. We found a significant decrease in superficial wound infections after surgery in the pNPWT group compared with the control group (24 versus 51%, p = 0.029, OR 0.30 (95% CI 0.10–0.90)). The number of incisional wound infections significantly decreased after the introduction of pNPWT (7 versus 48%, p < 0.001, OR 0.08 (95% CI 0.02–0.39)), while the number of subcutaneous abscesses was comparable (5 versus 4). In the pNPWT group, all wound infections involved contaminated operations, whereas an additional three patients had wound infections after clean surgery in the control group. The number of patients needing subcutaneous drainage was comparable in both groups (22 versus 24%). However, (the need for) opening of the wound with or without antibiotics was significantly decreased after the introduction of pNPWT (7 versus 48%, p < 0.001, OR 0.08 (95% CI 0.02–0.39). The need for postoperative intra-abdominal interventions was comparable in both groups, including two relaparotomies for anastomotic leakage in the control group and 9 and 8 radiologic drainages, respectively, in intervention and control group. Six patients in the control group needed NPWT due to wound dehiscence compared with seven patients in the pNPWT group needing prolonged NPWT.
Discussion
pNPWT on a closed midline laparotomy skin incision significantly reduced wound infections in patients undergoing major complex abdominal wall repair (24 versus 51%, p = 0.029, OR 0.30 (95% CI 0.10–0.90)), and even more for incisional wound infections only (7 versus 48%, p < 0.001, OR 0.08 (95% CI 0.02–0.39)). Although the number of intra-abdominal and radiologic subcutaneous interventions did not decrease, the number of wounds which spontaneously opened due to infection or were opened as part of incisional infection treatment was significantly decreased (p < 0.001). In this cohort, pNPWT failed to reduce other wound complications, emergency department visits and re-admissions.
As far as we know there are no randomized controlled trials published on pNPWT in abdominal surgery. We identified four controlled observational studies in patients undergoing ventral hernia repair [19,20,21,22]. Only two of them [20] found a significant benefit in reducing SSI. One study [20] involved large clean hernia repairs; another study [22] included both clean and clean-contaminated procedures. In contrast, Condé-Green et al. [19] found a significant difference in the number of wound complications as well as the number of skin dehiscence but failed to detect a difference in mere wound infections. In that study, all operations involved clean large ventral hernia repairs. The fourth study, Pauli et al. [21] focused on contaminated and infected hernias and do not find a difference in wound infections. Although the patient population in that study may seem comparable with our population, important differences exist when looking in more detail. Although Pauli et al. mentioned in their title ‘high-risk abdominal wall reconstructions’, they excluded patients undergoing anterior CST, patients with simultaneous colo- or enterocutaneous fistula takedown and patients undergoing simultaneous panniculectomy, and in particular these patients are at higher risk for SSI. In our study, almost half of the patients had one or more ECF and 91% underwent CST, therefore, representing a population at a much higher risk for wound infections. Hence, the discrepancy of their results with our findings may well be explained by the striking differences in included patient population. Recently, for all types of surgery a systematic review and meta-analysis on pNPWT has been performed [23]. An overall weighted average SSI rate of 6.61% in the pNPWT groups was found compared with 9.36% in the control groups, reflecting a relative reduction of 29.4%. A subgroup analysis of studies in abdominal surgery showed 13.54% SSI in the intervention group compared with 23.97% in the control group. Notably, the risk of SSI and subsequent SSI rates is much higher in complex and contaminated abdominal (wall) surgery such as encountered in the patients included in our study.
In our patient group, of which 75% of the patients underwent bowel resection and/or anastomosis, it can be difficult to distinguish between intra-abdominal complications due to anastomotic leakage or perforation and organ space surgical site infections due to wound complications. So far, it is unlikely that pNPWT has any influence on the intra-abdominal postoperative situation in closed fascia settings. In our opinion, the influence of pNPWT on intra-abdominal complications is negligible when the fascia is closed. As the aim of our study was to investigate the influence of pNPWT on wound infections, we chose to separately score intra-abdominal complications (bowel content leakage and intra-abdominal abscesses) and interventions for wound infections (superficial and deep). The first mentioned are more likely caused by anastomotic leakage, perforation or spillage of bowel content during the operation rather than due to wound complications. Moreover, we divided the superficial wound infections in subcutaneous abscesses and incisional wound infections. The reason is that the CDC SSI classification does not distinguish between these two different types of superficial infections. We found that a considerable number of patients in the pNPWT group had a subcutaneous abscess while not having an incisional SSI and, thus, a closed surgical incision wound. The reason might be that 91% of the patients underwent component separation technique, creating increased wound surface and dead space.
Although retrospective cohort studies are prone to selection bias, we tried to minimize bias by comparing our intervention cohort with a cohort of patients operated within the same time span. In some of the previous studies, the choice for pNPWT or conventional dressings was based on the preference of the surgeon, which might create a higher risk of bias. In addition, we did not find any significant differences in baseline characteristics, reflecting comparable study groups.
SSI cause an enormous burden on healthcare costs [24]. On the other hand, pNPWT also incurs additional costs. We identified three studies [25,26,27] on cost-effectiveness of pNPWT and these were all performed in a gynaecological population. Lewis et al. [26] found that the risk of wound complications must be reduced by 33% for pNPWT to achieve cost savings. For obese and morbid obese patients, required reduction was slightly less. Echebiri et al. [25] concluded that if surgical site infection rates are greater than 14%, pNPWT could be cost-beneficial depending on the degree of reduction in surgical site infections. At a surgical site infection rate of 30%, the rate must be reduced by 15% for pNPWT to become the preferred strategy. Although these studies involved a different patient group with clean surgery and, therefore, simply extrapolating these results cannot be done; these calculations do suggest that pNPWT is potentially cost-beneficial in our cohort.
This study is limited by the retrospective and non-randomized design. It can be difficult to score wound complications from a patient chart. Next to this, there might be an interpreter bias as the one who did the data extraction was not blinded for the therapy. Last but not least, not all patients had an equal follow-up. As we are a tertiary hospital, some patients living far away might have returned to other hospitals in the country if wound complications occurred. Nevertheless, all patients had outpatient follow-up at least once after 30 days, so the number of wound complications missed is probably limited to exceptional very late ones.
Closed incision prophylactic NPWT seems a promising solution to reduce the incidence of incisional wound infections in complex abdominal wall surgery. Randomized controlled trials are needed to estimate more precisely the value and cost-effectiveness of prophylactic NPWT in this high-risk setting.
References
Kanters AE, Krpata DM, Blatnik JA et al (2012) Modified hernia grading scale to stratify surgical site occurrence after open ventral hernia repairs. J Am Coll Surg 215:787–793
Slater NJ, Montgomery A, Berrevoet F et al (2014) Criteria for definition of a complex abdominal wall hernia. Hernia 18:7–17
Basta MN, Fischer JP, Kovach SJ (2015) Assessing complications and cost-utilization in ventral hernia repair utilizing biologic mesh in a bridged underlay technique. Am J Surg 209:695–702
Garvey PB, Martinez RA, Baumann DP, Liu J, Butler CE (2014) Outcomes of abdominal wall reconstruction with acellular dermal matrix are not affected by wound contamination. J Am Coll Surg 219:853–864
Pinell-White XA, Gruszynski M, Losken A (2014) Ventral hernia repair after bowel surgery. Ann Plast Surg 72:150–152
Kim H, Bruen K, Vargo D (2006) Acellular dermal matrix in the management of high-risk abdominal wall defects. Am J Surg 192:705–709
Itani KMF, Rosen M, Vargo D, Awad SS, Denoto G, Butler CE (2012) Prospective study of single-stage repair of contaminated hernias using a biologic porcine tissue matrix: the RICH Study. Surgery 152:498–505
Iacco A, Adeyemo A, Riggs T, Janczyk R (2014) Single institutional experience using biological mesh for abdominal wall reconstruction. Am J Surg 208:480–484
Rosen MJ, Krpata DM, Ermlich B, Blatnik JA (2013) A 5-year clinical experience with single-staged repairs of infected and contaminated abdominal wall defects utilizing biologic mesh. Ann Surg 257:991–996
Slater NJ, Bokkerink WJV, Konijn V, Bleichrodt RP, van Goor H (2015) Safety and durability of 1-stage repair of abdominal wall defects with enteric fistulas. Ann Surg 261:553–557
CDC (2015) Surgical site infection. CDC, London, pp 1–26
Allegranzi B, Bischoff P, de Jonge S et al (2016) New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 3099:1–16
Allegranzi B, Zayed B, Bischoff P et al (2016) New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 16:e288–e303
Stannard JP, Volgas DA, Stewart R, McGwin G, Alonso JE (2009) Negative pressure wound therapy after severe open fractures: a prospective randomized study. J Orthop Trauma 23:552–557
Canadian Agency for Drugs and Technologies in Health (2014) Negative pressure wound therapy for managing diabetic foot ulcers: a review of the clinical effectiveness, cost-effectiveness, and guidelines. CADTH Rapid Response Reports
Roberts DJ, Zygun DA, Grendar J et al (2012) Negative-pressure wound therapy for critically ill adults with open abdominal wounds. J Trauma Acute Care Surg 73:629–639
Meissner K (1999) Late radiogenic small bowel damage: guidelines for the general surgeon. Dig Surg 16:169–174
Borgquist O, Ingemansson R, Malmsjö M (2011) Individualizing the use of negative pressure wound therapy for optimal wound healing: a focused review of the literature. Ostomy Wound Manage 57:44–54
Condé-Green A, Chung TL, Holton LH et al (2012) Incisional negative-pressure wound therapy versus conventional dressings following abdominal wall reconstruction. Ann Plast Surg 71:1
Gassman A, Mehta A, Bucholdz E et al (2014) Positive outcomes with negative pressure therapy over primarily closed large abdominal wall reconstruction reduces surgical site infection rates. Hernia 19:273–278
Pauli EM, Krpata DM, Novitsky YW, Rosen MJ (2013) Negative pressure therapy for high-risk abdominal wall reconstruction incisions. Surg Infect 14:270–274
Soares KC, Baltodano PA, Hicks CW et al (2015) Novel wound management system reduction of surgical site morbidity after ventral hernia repairs: a critical analysis. Am J Surg 209:324–332
Semsarzadeh NN, Tadisina KK, Maddox J, Chopra K, Singh DP (2015) Closed incision negative-pressure therapy is associated with decreased surgical-site infections. Plast Reconstr Surg 136:592–602
de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB (2009) Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 37:387–397
Echebiri NC, McDoom MM, Aalto MM, Fauntleroy J, Nagappan N, Barnabei VM (2015) Prophylactic use of negative pressure wound therapy after cesarean delivery. Obstet Gynecol 125:299–307
Lewis LS, Convery PA, Bolac CS, Valea FA, Lowery WJ, Havrilesky LJ (2014) Cost of care using prophylactic negative pressure wound vacuum on closed laparotomy incisions. Gynecol Oncol 132:684–689
Tuffaha HW, Gillespie BM, Chaboyer W, Gordon LG, Scuffham PA (2015) Cost-utility analysis of negative pressure wound therapy in high-risk cesarean section wounds. J Surg Res 195:612–622
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.A. Boermeester reports institutional grants from J&J/Ethicon, Acelity/KCI, LifeCell, Ipsen, Baxter, Mylan, Bard, outside the submitted work; advisory board member of J&J/Ethicon, Acelity/KCI and LifeCell. The other authors do not declare any conflicts of interest.
Funding
None.
Ethical approval
The medical ethical committee of the hospital approved the study.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
The medical ethical committee waived the need of informed consent due to the retrospective design of the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
de Vries, F.E.E., Atema, J.J., Lapid, O. et al. Closed incision prophylactic negative pressure wound therapy in patients undergoing major complex abdominal wall repair. Hernia 21, 583–589 (2017). https://doi.org/10.1007/s10029-017-1620-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-017-1620-0