Abstract
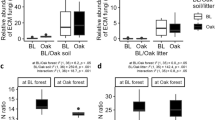
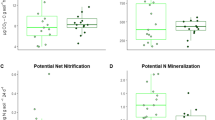
It has been proposed that ectomycorrhizal (EcM) fungi slow down decomposition by competing with free-living saprotrophs for organic nutrients and other soil resources (known as the “Gadgil effect”), thereby increasing soil carbon sequestration. As such, this Gadgil effect should depend on soil organic matter age and quality, but this remains unstudied. In addition, the Gadgil effect is not expected to occur in arbuscular mycorrhizal (AM) forests since AM fungi cannot access directly nutrients from soil organic matter, yet few direct comparisons between EcM and AM forests have been made. We performed a two-year reciprocal decomposition experiment of soil organic horizons (litter—L, fragmented—F, humic—H) in adjacent temperate deciduous forests dominated by EcM or AM trees. Mesh bags were made of different mesh sizes allowing or excluding ingrowth of external fungal hyphae, which are primarily mycorrhizal in these forests other than for the most recent superficial litter horizon. As expected, EcM stands stored more soil carbon (up to 20 cm depth) than AM stands. Also, organic matter originating from deeper horizons and from EcM stands was of lower quality (for example, higher lignin to nitrogen ratios) and decomposed more slowly. However, contrary to the Gadgil effect, organic matter exposed to external fungal hyphae (that is, primarily mycorrhizal) actually decomposed faster in both forest types, and this effect was strongest in EcM forests, particularly in the F horizon. Unexpectedly, organic matter decomposition was faster in EcM than in AM forests, regardless of organic matter origin. Overall, our study reinforces the view that temperate EcM forests store greater amounts of soil organic carbon than AM forests, but suggests that this is due to factors other than the Gadgil effect.



Similar content being viewed by others
Data Availability
Data are deposited in Zenodo: https://doi.org/10.5281/zenodo.3743507
References
Allison SD, Lu Y, Weihe C, Goulden ML, Martiny AC, Treseder KK, Martiny JBH. 2013. Microbial abundance and composition influence litter decomposition response to environmental change. Ecology 94:714–725.
Austin AT, Vivanco L, González-Arzac A, Pérez LI. 2014. There’s no place like home? An exploration of the mechanisms behind plant litter–decomposer affinity in terrestrial ecosystems. New Phytologist 204:307–314.
Averill C, Turner BL, Finzi AC. 2014. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505:543–545.
Bahram M, Peay KG, Tedersoo L. 2015. Local-scale biogeography and spatiotemporal variability in communities of mycorrhizal fungi. New Phytol 205:1454–1463.
Baldrian P. 2017. Forest microbiome: diversity, complexity and dynamics. FEMS Microbiol Rev 41:109–130.
Bardgett RD, Wardle DA. 2010. Aboveground-belowground linkages: Biotic interactions, ecosystem processes, and global change. OUP Oxford
Bélanger N, Côté B, Fyles JW, Courchesne F, Hendershot WH. 2004. Forest regrowth as the controlling factor of soil nutrient availability 75 years after fire in a deciduous forest of Southern Quebec. Plant and Soil 262:363–272.
Berg B, McClaugherty C. 2014. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration. 3rd ed. Berlin Heidelberg: Springer-Verlag https://www.springer.com/gp/book/9783642388200. Last accessed 23/08/2019
Bödeker ITM, Lindahl BD, Olson Å, Clemmensen KE. 2016. Mycorrhizal and saprotrophic fungal guilds compete for the same organic substrates but affect decomposition differently. Funct Ecol 30:1967–1978.
Brzostek ER, Dragoni D, Brown ZA, Phillips RP. 2015. Mycorrhizal type determines the magnitude and direction of root-induced changes in decomposition in a temperate forest. New Phytol 206:1274–1282.
Bunn RA, Simpson DT, Bullington LS, Lekberg Y, Janos DP. 2019. Revisiting the ‘direct mineral cycling’ hypothesis: arbuscular mycorrhizal fungi colonize leaf litter, but why? The ISME Journal 13:1891.
Carrillo Y, Dijkstra FA, LeCain D, Pendall E. 2016. Mediation of soil C decomposition by arbuscular mycorrizhal fungi in grass rhizospheres under elevated CO2. Biogeochemistry 127:45–55.
Carteron A, Beigas M, Joly S, Turner BL, Laliberté E. 2021. Temperate forests dominated by arbuscular or ectomycorrhizal fungi are characterized by strong shifts from saprotrophic to mycorrhizal fungi with increasing soil depth. Microb Ecol:1–14. https://doi.org/10.1007/s00248-020-01540-7
Carvalhais N, Forkel M, Khomik M, Bellarby J, Jung M, Migliavacca M, Μu M, Saatchi S, Santoro M, Thurner M, Weber U, Ahrens B, Beer C, Cescatti A, Randerson JT, Reichstein M. 2014. Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature 514:213–217.
Chagnon P-L, Bradley RL, Maherali H, Klironomos JN. 2013. A trait-based framework to understand life history of mycorrhizal fungi. Trends in Plant Science 18:484–491.
Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A, Wallander H, Stenlid J, Finlay RD, Wardle DA, Lindahl BD. 2013. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339:1615–1618.
Clemmensen KE, Finlay RD, Dahlberg A, Stenlid J, Wardle DA, Lindahl BD. 2015. Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol 205:1525–1536.
Clemmensen KE, Durling MB, Michelsen A, Hallin S, Finlay RD, Lindahl BD. 2021. A tipping point in carbon storage when forest expands into tundra is related to mycorrhizal recycling of nitrogen. Ecology Letters 24:1193–1204.
Colin G, Cooney JD, Carlsson DJ, Wiles DM. 1981. Deterioration of plastic films under soil burial conditions. J Appl Polym Sci 26:509–519.
Côté B, Fyles JW. 1994. Leaf litter disappearance of hardwood species of southern Québec: Interaction between litter quality and stand type. Écoscience 1:322–328.
Cotrufo MF, Soong JL, Horton AJ, Campbell EE, Haddix ML, Wall DH, Parton WJ. 2015. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nature Geoscience 8:776–779.
Courchesne F, Côté B, Fyles JW, Hendershot WH, Biron PM, Roy AG, Turmel M-C. 2005. Recent changes in soil chemistry in a forested ecosystem of southern Québec, Canada. Soil Science Society of America Journal 69:1298.
Craig ME, Turner BL, Liang C, Clay K, Johnson DJ, Phillips RP. 2018. Tree mycorrhizal type predicts within-site variability in the storage and distribution of soil organic matter. Global Change Biology 24:3317–3330.
Crowther TW, van den Hoogen J, Wan J, Mayes MA, Keiser AD, Mo L, Averill C, Maynard DS. 2019. The global soil community and its influence on biogeochemistry. Science 365:eaav0550.
Dickie IA, Xu B, Koide RT. 2002. Vertical niche differentiation of ectomycorrhizal hyphae in soil as shown by T-RFLP analysis. New Phytologist 156:527–535.
Dickie IA, Koele N, Blum JD, Gleason JD, McGlone MS. 2014. Mycorrhizas in Changing Ecosystems. Botany 92:149–160.
Dighton J, Thomas ED, Latter PM. 1987. Interactions between tree roots, mycorrhizas, a saprotrophic fungus and the decomposition of organic substrates in a microcosm. Biol Fert Soils 4:145–150.
Dixon RK, Solomon AM, Brown S, Houghton RA, Trexier MC, Wisniewski J. 1994. Carbon pools and flux of global forest ecosystems. Science 263:185–190.
Fanin N, Lin D, Freschet GT, Keiser AD, Augusto L, Wardle DA, Veen GF (Ciska). 2021. Home-field advantage of litter decomposition: from the phyllosphere to the soil. New Phytologist n/a. https://doi.org/10.1111/nph.17475
Fernandez CW, Kennedy PG. 2016. Revisiting the ‘Gadgil effect’: do interguild fungal interactions control carbon cycling in forest soils? New Phytol 209:1382–1394.
Fernandez CW, See CR, Kennedy PG. 2019. Decelerated carbon cycling by ectomycorrhizal fungi is controlled by substrate quality and community composition. New Phytologist 226:569–582.
Fisher FM, Gosz JR. 1986. Effects of trenching on soil processes and properties in a New Mexico mixed-conifer forest. Biol Fert Soils 2:35–42.
Frey SD. 2019. Mycorrhizal fungi as mediators of soil organic matter dynamics. Annu Rev Ecol Evol Syst 50:237–259.
Gadgil RL, Gadgil PD. 1971. Mycorrhiza and litter decomposition. Nature 233:133–133.
Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ. 2000. Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Global Change Biology 6:751–765.
Gui H, Hyde K, Xu J, Mortimer P. 2017. Arbuscular mycorrhiza enhance the rate of litter decomposition while inhibiting soil microbial community development. Scientific Reports 7:42184.
Handa IT, Aerts R, Berendse F, Berg MP, Bruder A, Butenschoen O, Chauvet E, Gessner MO, Jabiol J, Makkonen M, McKie BG, Malmqvist B, Peeters ETHM, Scheu S, Schmid B, van Ruijven J, Vos VCA, Hättenschwiler S. 2014. Consequences of biodiversity loss for litter decomposition across biomes. Nature 509:218–221.
Hättenschwiler S, Bretscher D. 2001. Isopod effects on decomposition of litter produced under elevated CO2, N deposition and different soil types. Global Change Biology 7:565–579.
Hättenschwiler S, Tiunov AV, Scheu S. 2005. Biodiversity and litter decomposition in terrestrial ecosystems. Annual Review of Ecology, Evolution, and Systematics 36:191–218.
He X, Critchley C, Ng H, Bledsoe C. 2004. Reciprocal N (15NH4+ or 15NO3−) transfer between nonN2-fixing Eucalyptus maculata and N2-fixing Casuarina cunninghamiana linked by the ectomycorrhizal fungus Pisolithus sp. New Phytologist 163:629–640.
Hodge A. 2017. Chapter 8 - Accessibility of inorganic and organic nutrients for mycorrhizas. In: Mycorrhizal Mediation of Soil. Elsevier. pp 129–48. https://www.sciencedirect.com/science/article/pii/B9780128043127000085
Hodge A, Campbell CD, Fitter AH. 2001. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297–299.
Jacob M, Viedenz K, Polle A, Thomas FM. 2010. Leaf litter decomposition in temperate deciduous forest stands with a decreasing fraction of beech (Fagus sylvatica). Oecologia 164:1083–1094.
Jacobs LM, Sulman BN, Brzostek ER, Feighery JJ, Phillips RP. 2018. Interactions among decaying leaf litter, root litter and soil organic matter vary with mycorrhizal type. Journal of Ecology 106:502–513.
Johnson D, Leake JR, Read DJ. 2001. Novel in-growth core system enables functional studies of grassland mycorrhizal mycelial networks. New Phytologist 152:555–562.
Kassambara A. 2018. ggpubr: ‘ggplot2’ Based Publication Ready Plots. https://CRAN.R-project.org/package=ggpubrhttps://CRAN.R-project.org/package=ggpubr
Keller AB, Phillips RP. 2019. Leaf litter decay rates differ between mycorrhizal groups in temperate, but not tropical, forests. New Phytologist 222:556–564.
Koide RT, Wu T. 2003. Ectomycorrhizas and retarded decomposition in a Pinus resinosa plantation. New Phytologist 158:401–407.
Kubartová A, Ranger J, Berthelin J, Beguiristain T. 2008. Diversity and decomposing ability of saprophytic fungi from temperate forest litter. Microb Ecol 58:98–107.
Kuzyakov Y. 2010. Priming effects: Interactions between living and dead organic matter. Soil Biology and Biochemistry 42:1363–1371.
Kyaschenko J, Clemmensen KE, Karltun E, Lindahl BD. 2017. Below-ground organic matter accumulation along a boreal forest fertility gradient relates to guild interaction within fungal communities. Ecol Lett 20:1546–1555.
Lal R. 2005. Forest soils and carbon sequestration. Forest Ecology and Management 220:242–258.
Lang AK, Jevon FV, Vietorisz CR, Ayres MP, Matthes JH. 2021. Fine roots and mycorrhizal fungi accelerate leaf litter decomposition in a northern hardwood forest regardless of dominant tree mycorrhizal associations. New Phytologist 230:316–326.
Leifheit EF, Verbruggen E, Rillig MC. 2015. Arbuscular mycorrhizal fungi reduce decomposition of woody plant litter while increasing soil aggregation. Soil Biology and Biochemistry 81:323–328.
Lenth R. 2019. emmeans: Estimated Marginal Means, aka Least-Squares Means. https://CRAN.R-project.org/package=emmeanshttps://CRAN.R-project.org/package=emmeans
Li Y, Veen GF (Ciska), Hol WHG, Vandenbrande S, Hannula SE, ten Hooven FC, Li Q, Liang W, Bezemer TM. 2020. ‘Home’ and ‘away’ litter decomposition depends on the size fractions of the soil biotic community. Soil Biology and Biochemistry 144:107783.
Lin G, McCormack ML, Ma C, Guo D. 2017. Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytologist 213:1440–1451.
Lin D, Dou P, Yang G, Qian S, Wang H, Zhao L, Yang Y, Mi X, Ma K, Fanin N. 2020. Home-field advantage of litter decomposition differs between leaves and fine roots. New Phytologist 227:995–1000.
Lindahl BD, Tunlid A. 2015. Ectomycorrhizal fungi – potential organic matter decomposers, yet not saprotrophs. New Phytol 205:1443–1447.
Lindahl BD, Ihrmark K, Boberg J, Trumbore SE, Högberg P, Stenlid J, Finlay RD. 2007. Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytologist 173:611–620.
Lindahl BD, de Boer W, Finlay RD. 2010. Disruption of root carbon transport into forest humus stimulates fungal opportunists at the expense of mycorrhizal fungi. The ISME Journal 4:872–881.
Lovett GM, Arthur MA, Crowley KF. 2016. Effects of calcium on the rate and extent of litter decomposition in a northern hardwood forest. Ecosystems 19:87–97.
Maillard F, Kennedy PG, Adamczyk B, Heinonsalo J, Buée M. 2021. Root presence modifies the long-term decomposition dynamics of fungal necromass and the associated microbial communities in a boreal forest. Molecular Ecology 30:1921–1935.
Makkonen M, Berg MP, Handa IT, Hättenschwiler S, van Ruijven J, van Bodegom PM, Aerts R. 2012. Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecology Letters 15:1033–1041.
Malik RJ. 2019. No “Gadgil effect”: Temperate tree roots and soil lithology are effective predictors of wood decomposition. Forest Pathology 49:e12506.
Malik RJ, Trexler RV, Eissenstat DM, Bell TH. 2020. Bark decomposition in white oak soil outperforms eastern hemlock soil, while bark type leads to consistent changes in soil microbial composition. Biogeochemistry 150:329–343.
McHale PJ, Mitchell MJ, Bowles FP. 1998. Soil warming in a northern hardwood forest: trace gas fluxes and leaf litter decomposition. Can J for Res 28:1365–1372.
Midgley MG, Brzostek E, Phillips RP. 2015. Decay rates of leaf litters from arbuscular mycorrhizal trees are more sensitive to soil effects than litters from ectomycorrhizal trees. Journal of Ecology 103:1454–1463.
Moore TR, Trofymow JA, Taylor B, Prescott C, Camiré C, Duschene L, Fyles J, Kozak L, Kranabetter M, Morrison I, Siltanen M, Smith S, Titus B, Visser S, Wein R, Zoltai S. 1999. Litter decomposition rates in Canadian forests. Global Change Biology 5:75–82.
Mujic AB, Durall DM, Spatafora JW, Kennedy PG. 2016. Competitive avoidance not edaphic specialization drives vertical niche partitioning among sister species of ectomycorrhizal fungi. New Phytologist 209:1174–1183.
Netherway T, Bengtsson J, Krab EJ, Bahram M. 2020. Biotic interactions with mycorrhizal systems as extended nutrient acquisition strategies shaping forest soil communities and functions. Basic and Applied Ecology. https://doi.org/10.1016/j.baae.2020.10.002.
Orwin KH, Kirschbaum MUF, St John MG, Dickie IA. 2011. Organic nutrient uptake by mycorrhizal fungi enhances ecosystem carbon storage: a model-based assessment. Ecology Letters 14:493–502.
Phillips RP, Brzostek E, Midgley MG. 2013. The mycorrhizal-associated nutrient economy: a new framework for predicting carbon–nutrient couplings in temperate forests. New Phytol 199:41–51.
Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC. 2012. nlme: Linear and nonlinear mixed effects models. R package version 3.
Prescott CE. 2005. Do rates of litter decomposition tell us anything we really need to know? Forest Ecology and Management 220:66–74.
R Core Team. 2018. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing https://www.R-project.org/
Read DJ, Leake JR, Perez-Moreno J. 2004. Mycorrhizal fungi as drivers of ecosystem processes in heathland and boreal forest biomes. Can J Bot 82:1243–1263.
Rosling A, Landeweert R, Lindahl BD, Larsson K-H, Kuyper TW, Taylor AFS, Finlay RD. 2003. Vertical distribution of ectomycorrhizal fungal taxa in a podzol soil profile. New Phytologist 159:775–783.
Schimel JP, Schaeffer SM. 2012. Microbial control over carbon cycling in soil. Front Microbiol 3:348.
Sietiö O-M, Santalahti M, Putkinen A, Adamczyk S, Sun H, Heinonsalo J. 2019. Restriction of plant roots in boreal forest organic soils affects the microbial community but does not change the dominance from ectomycorrhizal to saprotrophic fungi. FEMS Microbiol Ecol 95. https://academic.oup.com/femsec/article/95/9/fiz133/5554003. Last accessed 21/10/2019
Smith SE, Read DJ. 2008. Mycorrhizal Symbiosis. Academic Press.
Smith GR, Wan J. 2019. Resource-ratio theory predicts mycorrhizal control of litter decomposition. New Phytologist 223:1595–1606.
Soudzilovskaia NA, van der Heijden MGA, Cornelissen JHC, Makarov MI, Onipchenko VG, Maslov MN, Akhmetzhanova AA, van Bodegom PM. 2015. Quantitative assessment of the differential impacts of arbuscular and ectomycorrhiza on soil carbon cycling. New Phytol 208:280–293.
Soudzilovskaia NA, van Bodegom PM, Terrer C, van’t Zelfde M, McCallum I, McCormack ML, Fisher JB, Brundrett MC, de Sá NC, Tedersoo L. 2019. Global mycorrhizal plant distribution linked to terrestrial carbon stocks. Nat Commun 10:1–10.
Steidinger BS, Crowther TW, Liang J, Nuland MEV, Werner GDA, Reich PB, Nabuurs G, de-Miguel S, Zhou M, Picard N, Herault B, Zhao X, Zhang C, Routh D, Peay KG. 2019. Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature 569:404.
Sterkenburg E, Clemmensen KE, Ekblad A, Finlay RD, Lindahl BD. 2018. Contrasting effects of ectomycorrhizal fungi on early and late stage decomposition in a boreal forest. The ISME Journal 12:2187–2197.
Subke J-A, Voke NR, Leronni V, Garnett MH, Ineson P. 2011. Dynamics and pathways of autotrophic and heterotrophic soil CO2 efflux revealed by forest girdling. Journal of Ecology 99:186–193.
Taylor MK, Lankau RA, Wurzburger N. 2016. Mycorrhizal associations of trees have different indirect effects on organic matter decomposition. J Ecol 104:1576–1584.
Tedersoo L, Bahram M. 2019. Mycorrhizal types differ in ecophysiology and alter plant nutrition and soil processes. Biological Reviews 94:1857–1880.
Teste FP. 2008. Role of mycorrhizal networks in dry Douglas-fir forests. University of British Columbia https://open.library.ubc.ca/cIRcle/collections/ubctheses/24/items/1.0066342. Last accessed 21/10/2020
Teste FP, Karst J, Jones MD, Simard SW, Durall DM. 2006. Methods to control ectomycorrhizal colonization: effectiveness of chemical and physical barriers. Mycorrhiza 17:51–65.
Teste FP, Simard SW, Durall DM, Guy RD, Jones MD, Schoonmaker AL. 2009. Access to mycorrhizal networks and roots of trees: importance for seedling survival and resource transfer. Ecology 90:2808–2822.
Tisserant E, Malbreil M, Kuo A, Kohler A, Symeonidi A, Balestrini R, Charron P, Duensing N, dit Frey NF, Gianinazzi-Pearson V, Gilbert LB, Handa Y, Herr JR, Hijri M, Koul R, Kawaguchi M, Krajinski F, Lammers PJ, Masclaux FG, Murat C, Morin E, Ndikumana S, Pagni M, Petitpierre D, Requena N, Rosikiewicz P, Riley R, Saito K, Clemente HS, Shapiro H, van Tuinen D, Bécard G, Bonfante P, Paszkowski U, Shachar-Hill YY, Tuskan GA, Young JPW, Sanders IR, Henrissat B, Rensing SA, Grigoriev IV, Corradi N, Roux C, Martin F. 2013. Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. PNAS 110:20117–22.
van der Wal A, Geydan TD, Kuyper TW, de Boer W. 2013. A thready affair: linking fungal diversity and community dynamics to terrestrial decomposition processes. FEMS Microbiol Rev 37:477–494.
Veen GF (Ciska), Freschet GT, Ordonez A, Wardle DA. 2015. Litter quality and environmental controls of home-field advantage effects on litter decomposition. Oikos 124:187–95.
Verbruggen E, Pena R, Fernandez CW, Soong JL. 2017. Chapter 24 - Mycorrhizal interactions with saprotrophs and impact on soil carbon storage. In: Mycorrhizal Mediation of Soil. Elsevier. pp 441–60. https://www.sciencedirect.com/science/article/pii/B9780128043127000243
Wang Y, Li FY, Song X, Wang X, Suri G, Baoyin T. 2020. Changes in litter decomposition rate of dominant plants in a semi-arid steppe across different land-use types: Soil moisture, not home-field advantage, plays a dominant role. Agriculture, Ecosystems & Environment 303:107119.
Wickham H. 2016. ggplot2: Elegant graphics for data analysis. Springer-Verlag New York https://CRAN.R-project.org/package=ggplot2
Wickham H, Francois R, Henry L, Müller K. 2017. dplyr: A grammar of data manipulation. https://CRAN.R-project.org/package=dplyrhttps://CRAN.R-project.org/package=dplyr
Wiesmeier M, Urbanski L, Hobley E, Lang B, von Lützow M, Marin-Spiotta E, van Wesemael B, Rabot E, Ließ M, Garcia-Franco N, Wollschläger U, Vogel H-J, Kögel-Knabner I. 2019. Soil organic carbon storage as a key function of soils - A review of drivers and indicators at various scales. Geoderma 333:149–162.
Wurzburger N, Brookshire ENJ. 2017. Experimental evidence that mycorrhizal nitrogen strategies affect soil carbon. Ecology 98:1491–1497.
Xu J, Liu S, Song S, Guo H, Tang J, Yong JWH, Ma Y, Chen X. 2018. Arbuscular mycorrhizal fungi influence decomposition and the associated soil microbial community under different soil phosphorus availability. Soil Biology and Biochemistry 120:181–190.
Zak DR, Pellitier PT, Argiroff W, Castillo B, James TY, Nave LE, Averill C, Beidler KV, Bhatnagar J, Blesh J, Classen AT, Craig M, Fernandez CW, Gundersen P, Johansen R, Koide RT, Lilleskov EA, Lindahl BD, Nadelhoffer KJ, Phillips RP, Tunlid A. 2019. Exploring the role of ectomycorrhizal fungi in soil carbon dynamics. New Phytologist 223:33–39.
Zhu W, Ehrenfeld JG. 1996. The effects of mycorrhizal roots on litter decomposition, soil biota, and nutrients in a spodosolic soil. Plant Soil 179:109–118.
Acknowledgements
We would like to thank the staff from the Station de biologie des Laurentides (SBL) of Université de Montréal for facilitating the field work. Funding, including scholarships to AC, was provided by Discovery Grants to EL (RGPIN-2014-06106, RGPIN-2019-04537) by the Natural Sciences and Engineering Research Council of Canada (NSERC) as well as a “Nouveau Chercheur” grant (2016-NC-188823) by the Fonds de recherche du Québec sur la Nature et technologies (FRQNT). AC would like to sincerely thank the following institutions for providing generous scholarships: FRQNT (Dossier 272522), Institut de recherche en biologie végétale, Centre de la science de la biodiversité du Québec, Centre d'étude de la forêt and Université de Montréal through the "Bourse d'excellence Hydro-Québec."
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Author contributions: EL and AC conceived the ideas and designed methodology; AC and FC collected and analyzed the data; AC and EL interpreted the results; AC led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carteron, A., Cichonski, F. & Laliberté, E. Ectomycorrhizal Stands Accelerate Decomposition to a Greater Extent than Arbuscular Mycorrhizal Stands in a Northern Deciduous Forest. Ecosystems 25, 1234–1248 (2022). https://doi.org/10.1007/s10021-021-00712-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-021-00712-x