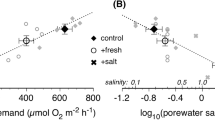
Abstract
Saltwater incursion carries high concentrations of sea salts, including sulfate, which can alter anaerobic microbial processes and plant community composition of coastal freshwater marshes. We studied these phenomena in a recently restored wetland on the coastal plain of North Carolina. We measured water inundation patterns, porewater chemistry, microbial process rates, plant tissue chemistry and iron plaque on plant roots, and quantified plant community composition across a hydrologic and salinity gradient to understand the potential interactions between saltwater incursion and changes in microbial processes and plant communities. Plant communities showed no obvious response to incursion, but were structured by inundation patterns and plant growth form (for example, graminoid versus forb). Saltwater incursion increased chloride and sulfate concentrations in surface and porewater, and drove resulting spatial patterns in anaerobic microbial metabolism rates. Plots experiencing saltwater incursion had higher sulfate reduction rates and were dominated by graminoid plant species (for example, sedges, rushes, and grasses). Graminoid plant species’ roots had greater iron plaque formation than forb and submerged species, indicative that graminoid plant species are supplying more oxygen to the rhizosphere, potentially influencing microbial metabolism. Future studies should focus on how plant and microbial communities may respond to saltwater incursion at different time scales, and on parsing out the influence that plants and microbes have on each other as freshwater wetlands experience sea level rise.







Similar content being viewed by others
References
Adams DA. 1963. Factors influencing vascular plant zonation in North Carolina salt marshes. Ecology 44:445–56.
Ardón M, Morse JL, Doyle MW, Bernhardt ES. 2010. The water quality consequences of restoring wetland hydrology to a large agricultural watershed in the southeastern coastal plain. Ecosystems 13:1060–78.
Ardón M, Morse JL, Colman BP, Bernhardt ES. 2013. Drought-induced saltwater incursion leads to increased wetland nitrogen export. Glob Change Biol 19:2976–85.
Armstrong W. 1964. Oxygen diffusion from the roots of some British bog plants. Nature 204:801–2.
Baldwin AH, Egnotovich MS, Clarke E. 2001. Hydrologic change and vegetation of tidal freshwater marshes: field, greenhouse, and seed-bank experiments. Wetlands 21:519–31.
Baldwin DS, Rees GN, Mitchell AM et al. 2006. The short-term effects of salinization on anaerobic nutrient cycling and microbial community structure in sediment from a freshwater wetland. Wetlands 26:455–64.
Bertness MD, Ellison AM. 1987. Determinants of pattern in a New England salt marsh community. Ecol Monogr 57:129–47.
Braun-Blanquet J. 1964. Pflanzensoziologie. Berlin: Springer.
Brettar I, Rheinheimer G. 1991. Denitrification in the central Baltic—evidence for H2S-oxidation as motor of denitrification at the oxic-anoxic interface. Mar Ecol Prog Ser 77:157–69.
Brix H. 1993. Macrophyte-mediated oxygen transfer in wetlands: transport mechanisms and rates. In: Moshiri GA, Ed. Constructed wetlands for water quality improvement. Boca Raton: Lewis Publishers. p 391–8.
Burgin AJ, Hamilton SK, Jones SE, Lennon JT. 2012. Denitrification by sulfur-oxidizing bacteria in a eutrophic lake. Aquat Microb Ecol 66:283–93.
Chambers LG, Osborne TZ, Reddy RK. 2013. Effect of salinity-altering pulsing events on soil organic carbon loss along an intertidal wetland gradient: a laboratory experiment. Biogeochemistry 115:363–83.
Craft CB. 2007. Freshwater input structures soil properties, vertical accretion, and nutrient accumulation of Georgia and U.S. tidal marshes. Limnol Oceanogr 52:1220–30.
Craft C, Clough J, Ehman J, Joye S, Park R, Pennings S, Guo HY, Machmuller M. 2009. Forecasting the effects of accelerated sea-level rise on tidal marsh ecosystem services. Front Ecol Environ 7:73–8.
Crain CM, Silliman BR, Bertness SL, Bertness MD. 2004. Physical and biotic drivers of plant distribution across estuarine salinity gradients. Ecology 85:2539–49.
Crawford RMM. 1993. Root survival in flooded soils. In: Gore A, Ed. Mires, swamp, bog, fen and moor, ecosystems of the world, Vol. 4A. Amsterdam: Elsevier Science. p 257–83.
Crump BC, Hopkinson CS, Sogin ML, Hobbie JE. 2004. Microbial biogeography along an estuarine salinity gradient: combined influences of bacterial growth and residence time. Appl Environ Microbiol 70:1494–505.
DeLaune RD, White JR. 2012. Will coastal wetlands continue to sequester carbon in response to an increase in global sea level?: a case study of the rapidly subsiding Mississippi river deltaic plain. Clim Change 110:297–314.
Dodla SK, Wang JJ, Delaune RD, Cook RL. 2008. Denitrification potential and its relation to organic carbon quality in three coastal wetland soils. Sci Total Environ 407:471–80.
Emerson D, Weiss JV, Megonigal JP. 1999. Iron-oxidizing bacteria are associated with ferric hydroxide precipitates (Fe-plaque) on the roots of wetland plants. Appl Environ Microbiol 65:2758–61.
Engels JG, Jensen K. 2009. Role of biotic interactions and physical factors in determining the distribution of marsh species along an estuarine salinity gradient. Oikos 119:679–85.
Fossing H, Jørgensen BB. 1989. Chromium reduction method of bacterial sulfate reduction in sediments: measurement reduction of a single-step chromium method evaluation. Biogeochemistry 8:205–22.
Golterman HL, Clymo RS, Eds. 1969. Methods for chemical analysis of sulphide in fresh waters, Vol. 8Oxford: Blackwell Scientific.
Hines ME, Knollmeyer SL, Tugel JB. 1989. Sulfate reduction and other sedimentary biogeochemistry in a northern New England salt marsh. Limnol Oceanogr 34:578–90.
Hopfensperger KN, Kaushal SS, Findlay SEG, Cornwell JC. 2009. Influence of plant communities on denitrification in a tidal freshwater marsh of the Potomac River, United States. J Environ Qual 38:618–26.
Hopkinson CS, Cai WJ, Hu X. 2012. Carbon sequestration in wetland dominated coastal systems—a global sink of rapidly diminishing magnitude. Curr Opin Environ Sustain 4:186–94.
Howarth RW, Teal JM. 1979. Sulfate reduction in a New England salt marsh. Limnol Oceanogr 24:999–1013.
Howarth RW, Giblin A. 1983. Sulfate reduction in the salt marshes at Sapelo Island, Georgia. Limnol Oceanogr 28:70–82.
Jackson ML. 1958. Soil chemical analysis—advanced course. Madison, Wisconsin: University of Wisconsin.
Kayranli B, Scholz M, Mustafa A, Hedmark Å. 2010. Carbon storage and fluxes within freshwater wetlands: a critical review. Wetlands 30:111–24.
Keller JK, Wolf AA, Weisenhom PB, Drake BG, Megonigal JP. 2009. Elevated CO2 affects porewater chemistry in a brackish marsh. Biogeochemistry 96:101–17.
Koch MS, Mendelssohn IA. 1989. Sulfide as a soil phytotoxin—differential responses in 2 marsh species. J Ecol 77:565–78.
Kostka JE, Gribsholt B, Petrie E, Dalton D, Skeleton H, Kristensen E. 2002. The rates and pathways of carbon oxidation in bioturbated saltmarsh sediments. Limnol Oceanogr 47:230–40.
Lovley DR, Phillips EJP. 1986. Organic matter mineralization with reduction of ferric iron in anaerobic sediments organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol 51:683–9.
Lowe KL, Dichristina TJ, Roychoudhury AN, Van Cappellen P. 2000. Microbiological and geochemical characterization of microbial Fe(III) reduction in salt marsh sediments. Geomicrobiol J 17:163–76.
McCune B, Grace JB. 2002. Analysis of ecological communities. Gleneden Beach: MjM Software Design.
Meehl GA, Covey C, Delworth T et al. 2007. The WCRP CMIP3 multimodel dataset—a new era in climate change research. Bull Am Meteorol Soc 88:1383–94.
Mendelssohn IA, Kleiss BA, Wakeley JS. 1995. Factors controlling the formation of oxidized root channels: a review. Wetlands 15:37–46.
Meyer JL, Sale MJ, Mulholland PJ et al. 1999. Impacts of climate change on aquatic ecosystem functioning and health. J Am Water Resour Assoc 35:1373–86.
Michener WK, Blood ER, Bildstein KL et al. 1997. Climate change, hurricanes and tropical storms, and rising sea level in coastal wetlands. Ecol Appl 7:770–801.
Morse JL, Ardón M, Bernhardt ES. 2012. Greenhouse gas fluxes in southeastern U.S. coastal plain wetlands under contrasting land uses. Ecol Appl 22:264–80.
Mulholland PJ, Best GR, Coutant CC et al. 1997. Effects of climate change on freshwater ecosystems of the southeastern United States and the Gulf Coast of Mexico. Hydrol Process 11:949–70.
Neubauer SC, Emerson D, Megonigal JP. 2002. Life at the energetic edge: kinetics of circumneutral iron oxidation by lithotrophic iron-oxidizing bacteria isolated from the wetland-plant rhizosphere. Appl Environ Microbiol 68:3988–95.
Neubauer SC, Givler K, Valentine SK, Megonigal JP. 2005. Seasonal patterns and plant-mediated controls of subsurface wetland biogeochemistry. Ecology 86:3334–44.
Neubauer SC, Toledo-Duran GE, Emerson D, Megonigal JP. 2007. Returning to their roots: iron-oxidizing bacteria enhance short-term plaque formation in the wetland-plant rhizosphere. Geomicrobiol J 24:65–73.
Neubauer SC, Franklin RB, Berrier DJ. 2013. Saltwater intrusion into tidal freshwater marshes alters the biogeochemical processing of organic carbon. Biogeosci Discuss 10:10685–720.
Noe GB, Zedler JB. 2001. Spatio-temporal variation of salt marsh seedling establishment in relation to the abiotic and biotic environment. J Veg Sci 12:61–74.
Odum WE. 1988. Comparative ecology of tidal fresh-water and salt marshes. Annu Rev Ecol Syst 19:147–76.
Palmer MW. 1993. Putting things in even better order—the advantages of canonical correspondence—analysis. Ecology 74:2215–30.
Pennings SC, Bestor G, Bertness MD. 2005. Plant zonation in low-latitude salt marshes: disentangling the roles of flooding, salinity and com-petition. J Ecol 93:159–67.
Perry JEIII, Hershner C. 1999. Temporal changes in the vegetation pattern in a tidal freshwater marsh. Wetlands 19:90–9.
Poffenbarger HJ, Needelman BA, Megonigal JP. 2011. Salinity influence on methane emissions from tidal marshes. Wetlands 31:831–42.
Roden EE, Wetzel RG. 1996. Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated wetland sediments. Limnol Oceanogr 41:1733–48.
Santoro AE. 2010. Microbial nitrogen cycling at the saltwater-freshwater interface. Hydrogeol J 18:187–202.
Scharfy D, Funk A, Venterink HO, Gusewell S. 2011. Invasive forbs differ functionally from native graminoids, but are similar to native forbs. New Phytol 189:818–28.
Schuyler AE, Andersen SB, Kolaga VJ. 1993. Plant zonation in the tidal portion of the Delaware River. Proc Acad Nat Sci Phila 144:263–6.
Sharpe PJ, Baldwin AH. 2009. Patterns of wetland plant species richness across estuarine gradients of Chesapeake Bay. Wetlands 29:225–35.
Sharpe PJ, Baldwin AH. 2012. Tidal marsh plant community response to sea-level rise: a mesocosm study. Aquat Bot 101:34–40.
Simpson RL, Good RE, Leck MA, Whigham DF. 1983. The ecology of freshwater tidal wetlands. Bioscience 33:255–9.
Snow AA, Vince SW. 1984. Plant zonation in an Alaskan salt marsh: II. An experimental study of the role of edaphic conditions. J Ecol 72:669–84.
Stanley EH, Ward AK. 2010. Effects of vascular plants on seasonal pore water carbon dynamics in a lotic wetland. Wetlands 30:889–900.
Steudel B, Hautier Y, Hector A, Kessler M. 2011. Diverse marsh plant communities are more consistently productive across a range of different environmental conditions through functional complementarity. J Appl Ecol 48:1117–24.
Sundby B, Vale C, Cacador I, Catarino F, Madureira MJ, Caetano M. 1998. Metal-rich concretions on the roots of salt marsh plants: mechanism and rate of formation. Limnol Oceanogr 43:245–52.
Sundby B, Vale C, Caetano M, Luther GWIII. 2003. Redox chemistry in the root zone of a salt marsh sediment in the Tagus Estuary, Portugal. Aquat Geochem 9:257–71.
Sutton-Grier A, Keller JK, Koch R, Gilmour C, Megonigal JP. 2011. Electron donors and acceptors influence anaerobic soil organic matter mineralization in tidal marshes. Soil Biol Biochem 43:1576–83.
Taylor GJ, Crowder AA. 1983. Use of the DCB technique for extraction of hydrous iron oxides from roots of wetland plants. Am J Bot 70:1254–7.
ter Braak CJF. 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167–79.
Wang FY, Chapman PM. 1999. Biological implications of sulfide in sediment—a review focusing on sediment toxicity. Environ Toxicol Chem 18:2526–32.
Weston NB, Porubsky WP, Samarkin VA, Erickson M, Macavoy SE, Joye SB. 2006. Porewater stoichiometry of terminal metabolic products, sulfate, and dissolved organic carbon and nitrogen in estuarine intertidal creek-bank sediments. Biogeochemistry 77:375–408.
Weston NB, Vile MA, Neubauer SC, Velinsky DJ. 2011. Accelerated microbial organic matter mineralization following salt-water intrusion into tidal freshwater marsh soils. Biogeochemistry 102:135–51.
Westrich JT, Berner RA. 1984. The role of sedimentary organic-matter in bacterial sulfate reduction—the G model tested. Limnol Oceanogr 29:236–49.
Yang JX, Liu Y, Ye ZH. 2012. Root-induced changes of pH, Eh, Fe(II) and fractions of Pb and Zn in rhizosphere soils of four wetland plants with different radial oxygen losses. Pedosphere 22:518–27.
Acknowledgements
We thank Anna Fedders, Medora Burke-Scoll, and Sarah Harvey for field and lab assistance, and Brooke Hassett for lab assistance. Marcelo Ardón provided long-term water level and conductivity datasets, and thoughtful review. Thanks to Emily Bernhardt for providing an insightful review of the paper. Funding for KNH provided by NSF DEB 1021039. Funding for all others provided by NSF DEB 1216916.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
KNH and AJB conceived and designed the overall study. AJB and VAS performed anaerobic microbial metabolism work. AMH performed site chemistry and inundation work. KNH performed plant chemistry and community work and statistical analyses. KNH wrote the paper with substantial input and edits from AJB, AMH, and VAS.
Rights and permissions
About this article
Cite this article
Hopfensperger, K.N., Burgin, A.J., Schoepfer, V.A. et al. Impacts of Saltwater Incursion on Plant Communities, Anaerobic Microbial Metabolism, and Resulting Relationships in a Restored Freshwater Wetland. Ecosystems 17, 792–807 (2014). https://doi.org/10.1007/s10021-014-9760-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-014-9760-x