Abstract
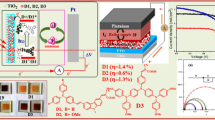
The auxiliary acceptors of metal complexes can easily regulate the electron-withdrawing ability of electron acceptor and adjust the balance of push–pull electron of D-A-π-A motif dye sensitizers. Two series of the D-(A-π-A)2 motif dye sensitizer (BDTT-im-Co, BDTT-im-Cu, BDTT-im-Zn, BDTT-im-Cd and FL-im-Co, FL-im-Cu, FL-im-Zn, FL-im-Cd) which use metal complexes of pyridineimine derivative as auxiliary acceptor were designed, synthesized, and characterized. The photovoltaic test of eight complex-based dye sensitized solar cells (DSSCs) showed that the short-circuit photocurrent density (JSC) and the power conversion efficiency (PCE) of two series dye sensitizers are sequentially increased. BDTT-im-Cd have the highest JSC of 15.58 mA cm−2 and the PCE of 9.13% under AM 1.5 irradiation, which may be due to that electron-withdrawing ability of the auxiliary acceptor metal complexes of pyridineimine derivative can be changed by the strength of coordination bond of the complexes. They also show good terminal stability with decomposition temperatures (Td) higher than 300 °C.







Similar content being viewed by others

Data availability
The data that support the findings of this study are available in the supplementary material of this article.
References
O’Regan B, Grätzel M (1991) Nature 353:737–740. https://doi.org/10.1021/j100179a001
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H (2010) Chem Rev 110:6595–6663. https://doi.org/10.1021/cr900356p
Grätzel MJ (2003) Photochem Photobio C Photochem Rev 4:145–153. https://doi.org/10.1016/S1389-5567(03)00026-1
Grätzel M, Janssen RAJ, Mitzi DB, Sargent E (2012) Nature 488:304–312. https://doi.org/10.1038/nature11476.
Sil MC, Sudhakar V, Mele MK et al (2017) ACS Appl Mater Interfaces 9(40):34875–34890. https://doi.org/10.1021/acsami.7b09010
Imahori H, Umeyama T (2009) J Phys Chem C 113(21):9029–9039. https://doi.org/10.1021/jp9007448
Zhu WH, Wu YZ, Wang ST et al (2011) Adv Funct Mater 21:756–763. https://doi.org/10.1002/adfm.201001801
Zhou H, Ji JM, Kang SH et al (2018) J Mater Chem C (2018) 7(10). https://doi.org/10.1039/C7TC03784H
Aono CM, Coutinho-Neto MD, Miotto R et al (2018) J Phys Chem C 122(48). https://doi.org/10.1021/acs.jpcc.8b09372
Cui Y, Wu YZ, Lu XF, Zhang X et al (2011) Chem Mater 23:4394–4401. https://doi.org/10.1021/cm202226j
Mao J, Guo F, Ying W, Wu W et al (2012) Chem Asian J 7:982–991. https://doi.org/10.1002/asia.201100967
Yen Y, Lee C, Hsu C, Chou H et al (2013) Chem Asian J 8:809–816. https://doi.org/10.1002/asia.201201173
Wu YZ, Zhang X, Li WQ, Wang ZS et al (2012) Adv Energy Mater 2:149–156. https://doi.org/10.1002/aenm.201100341
Wu YZ, Marszalek M, Zakeeruddin SM, Zhu WH (2012) Energy Environ Sci 5:8261–8272. https://doi.org/10.1039/C2EE22108J
Zhu HB (2014) Sustainable. Chem Eng 2:1026–1034. https://doi.org/10.1021/sc500035j
Chen L, Li X, Ying W, Zhang X et al (2013) Eur J Org Chem 1770–1780. https://doi.org/10.1002/ejoc.201201424
Li WQ, Wu YZ, Zhang Q, Tian H, Zhu WH (2012) ACS Appl Mater Interfaces 4:1822–1830. https://doi.org/10.1021/am3001049
Qu SY, Wu WJ, Hua JL, Kong C et al (2010) J Phys Chem C 114:1343–1349. https://doi.org/10.1021/jp909786k
Qu SY, Qin C, Islam A, Hua JL et al (2012) Chem Asian J 7:2895–2903. https://doi.org/10.1002/asia.201200648
Pei K, Wu YZ, Wu WJ, Zhang Q et al (2012) Chem Eur J 18:8190–8200. https://doi.org/10.1002/chem.201103542
Pei K, Wu YZ, Islam A, Zhang Q et al (2013) ACS Appl Mater Interfaces 5:4986–4995. https://doi.org/10.1021/am400754d
Pei K, Wu YZ, Islam A, Zhu SQ et al (2014) J Phys Chem C 118:16552–16561. https://doi.org/10.1021/jp412259t
Chen X et al (2017) Dyes Pigm 139:420–430. https://doi.org/10.1016/j.dyepig.2016.12.053
Pei K, Wu YZ, Li H, Geng ZY, Tian H, Zhu WH (2015) ACS Appl Mater Interfaces 7:5296–5304. https://doi.org/10.1002/asia.201200648
Qu SY, Qin C, Islam A, Wu YZ, Zhu WH et al (2012) Chem Commun 48:6972–6974. https://doi.org/10.1039/C2CC31998E
Patel DGD, Feng F, Ohnishi Y, Abboud KA et al (2012) J Am Chem Soc 134:2599–2612. https://doi.org/10.1021/ja207978v
Mathew S, Yella A, Gao P et al (2014) Nature Chem 6(3):242–247. https://doi.org/10.1038/nchem.1861
Wu YZ, Zhu WH, Zakeeruddin SM et al (2015) ACS Appl Mater Interfaces 7(18):9307–9318. https://doi.org/10.1021/acsami.5b02475
Abdul M, Maryam S, Salah UK, Naeem A (2020) Int J Quantum Chem 120(15):e26253. https://doi.org/10.1002/qua.26253
Zheng HL, Rui JM, Yi QX, Fan N et al (2020) Small Struct 2000052. https://doi.org/10.1002/adma.202005942
Sun ZD, He MF, Kadali C, Ju XH (2020) Mater Chem Phys 248:122943. https://doi.org/10.1016/j.matchemphys.2020.122943
Zhang HP, Wu XM, Tang SY, Zhong CF et al (2021) Appl Organomet Chem 35:e6220. https://doi.org/10.1002/aoc.6220
Chen X, Liao YL, Liu Y et al (2016) Dyes Pigm 139:420–430. https://doi.org/10.1016/j.dyepig.2016.12.053
Ito S, Liska P, Comte P, Gratzel M et al (2005) Chem Commun 4351–4353. https://doi.org/10.1039/B505718C
Mikroyannidis JA (1995) Polymer 36(6):1287–1293. https://doi.org/10.1016/0032-3861(95)93932-C
Zhou Y, Zhong CF, He Y et al (2009) J Inorg Organomet Polym Mater 19(3):328–334. https://doi.org/10.1007/s10904-009-9266-8
James CB, William CK (1975) Inorg Chem 14:2020–2021. https://doi.org/10.1021/ic50150a064
Funding
We received financial support from the Open Project Program of Key Laboratory of Environmentally Friendly Chemistry and Applications of Ministry of Education, China (Grant No. 19HJYH10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tian, Y., Wang, K., Wu, X. et al. Novel metal complexes of pyridineimine derivative used as auxiliary electron acceptor of D-(A-π-A)2 motif dye sensitizer: synthesis and photovoltaic application. J Solid State Electrochem 26, 719–727 (2022). https://doi.org/10.1007/s10008-022-05119-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-022-05119-9