Abstract
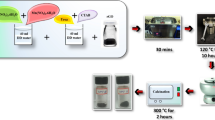
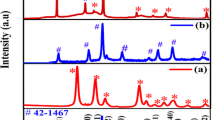
In the present investigation, we have synthesized Ni3V2O8@GO composite by co-precipitation method and designed as a new anode material for supercapacitor applications. The phase formation and surface morphology of the Ni3V2O8 and Ni3V2O8@GO composite were confirmed by XRD and FE-SEM measurements, respectively. It was observed that the synthesized Ni3V2O8@GO composite was found to have a small stone-like particle morphology. The electrochemical behavior of Ni3V2O8@GO composite as an electrode material for supercapacitor application was examined by electrochemical techniques. The obtained results showed that the Ni3V2O8@GO composite electrode exhibits maximum specific capacitance value 547 Fg−1 at a scan rate of 5 mVs−1 which was much superior than that of pure Ni3V2O8 (118 Fg−1). The improved electrochemical behavior of the Ni3V2O8@GO composite may be due to its well crystalline nature and also offers more active sites for Faradic reactions, good conductivity, and rapid diffusion of the electrolyte ions. Furthermore, the fabricated Ni3V2O8@GO composite electrode possessed an outstanding cyclic stability and nearly 95% of original specific capacitance value was sustained after 1500 charge-discharge cycles at 5 Ag−1. This indicates that shaped the spherical Ni3V2O8@GO composite possessed excellent electrochemical property that favors it as an auspicious electrode candidates for supercapacitor applications.











Similar content being viewed by others
References
Wang YG, Xia YY (2006) Electrochemical capacitance characterization of NiO with ordered mesoporous structure synthesized by template SBA -15. Electrochim Acta 51:3223–3227
Zhao DD, MW X, Zhou WJ, Zhang J, Li HL (2008) Preparation of ordered mesoporous nickel oxide film electrodes via lyotropic liquid crystal tempated electrodeposition route. Electrochim Acta 53:2699–2705
Xiong SL, Yuan CZ, Zhang XG, Xi BJ, Qian YT (2009) Controllable synthesis of mesoporous Co3O4 nanostructures with tunable morphology for application in supercapacitors. J Chem Eur 15:5320–5326
Pang H, Yan Z, Wang W, Chen J, Zhang JS, Zheng HH (2012) Facile fabrication of NH4CoPO4·H2O nano/microstructures and their primarily application as electrochemical supercapacitor. Nano 4:5946–5953
Pang H, Liu YY, Li J, Ma YH, Li GC, Ai YN, Chen J, Zhang JS, Zheng HH (2013) Cobalt phosphite micro architectures assembled by ultra long nano ribbons and their application as effective electrochemical capacitor electrode materials. Nano 5:503–507
Pang H, Yan Z, Ma Y, Li G, Chen J, Zhang J, Du W, Li S (2013) Cobalt pyrophosphate nano/microstructures as promising electrode materials of supercapacitor. J Solid State Electrochem 17:1383–1391
Theerthagiri J, Thiagarajan K, Senthilkumar B, Khan Z, Senthil RA, Arunachalam P, Madhavan J, Ashokkumar M (2017) Synthesis of hierarchical cobalt phosphate nanoflakes and their enhanced electrochemical performances for supercapacitor applications. Chem Select 2:201–210
Trasatti S, Kurzweil R (1994) Electrochemical supercapacitors as versatile energy stores. Platin Met Rev 38:46
ZY L, Yang Q, Zhu W, Chang Z, Liu JF, Sun XM, Evans DG, Duan X (2012) Hierarchical Co3O4@Ni-Co-O supercapacitor electrodes with ultrahigh specific capacitance per area. Nano Res 5:369–378
Wang GX, Cai J, HF X, Lu L, Zhao H (2014) Enhanced capacitance of a NiO electrode prepared in the magnetic field. J Appl Electrochem 44:391–398
Xu H, Zhuang JX, Li JL, Zhang JL, HL L (2014) Liquid precipitation synthesis of Co3O4 for high performance electrochemical capacitors. Ionics 20:489–494
Lawes G, Harris AB, Kimura T, Rogado N, Cava RJ, Aharony A, Wohlman OE, Yildirim T, Kenzelmann M, Broholm C, Ramirez AP (2005) Magnetically driven ferroelectric order in Ni3V2O8. Phys Rev Lett 95:087205
Wang DF, Tang JW, Zou ZG, Ye J (2005) Photophysical and photocatalytic properties of a new series of visible-light-driven photocatalysts M3V2O8 (M = Mg, Ni, Zn). Chem Mater 17:5177–5182
He ZZ, Yamaura J, Ueda Y (2008) Morphologies of Ni3V2O8 single crystals. Cryst Growth Des 8:799–801
Zhaorigetu B, Li WZ, Kieffer R, Xu HY (2002) Synergetic effect between NiO and Ni3V2O8 in propane oxidative dehydrogenation. React Kinet Catal Lett 75:275–287
Zheng S, ZS W, Wang S, Xiao H, Zhou F, Sun C, Bao X, Cheng HM (2017) Graphene based materials for high-voltage and high-energy asymmetric supercapacitors. Energy Storage Mater 6:70–97
Das AK, Sahoo S, Arunachalam P, Zhang S, Shim JJ (2016) Facile synthesis of Fe3O4 nanorod decorated reduced graphene oxide (RGO) for supercapacitor application. RSC Adv 6:107057–107064
Justin P, Meher SK, Raob GR (2010) Tuning of capacitance behavior of NiO using anionic, cationic and nonionic surfactants by hydrothermal synthesis. J Phy Chem 114:5203–5210
Wang C, Fang D, Wang H, Cao Y, Xu W, Liu X, Luo Z, Li G, Jiang M, Xiong C (2016) Uniform nickel vanadate (Ni3V2O8) nanowire arrays organized by ultrathin nanosheets with enhanced lithium storage properties. Sci Rep 6:20826
Prusty B, Adhikary MC, Das CK (2015) Supercapacitor electrode material based on nickel vanadium oxide. J A Chem Sci 6(2):91–95
Liu MC, Kong LB, Kang L, Li X, Walsh FC, Xing M, Lu C, Maa XJ, Luob YC (2014) Synthesis and characterization of M3V2O8 (M = Ni or Co) based nanostructures: a new family of high performance pseudocapacitive materials. J Mater Chem A 2:4829–5150
Hummers JR, S W, Offeman ER (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339–1339
Senthilkumar B, Vijaya Sankar K, Kalai Selvan R, Danielle M, Manickam M (2013) Nano α-NiMoO4 as a new electrode for electrochemical supercapacitors. RSC Adv 3:352–357
Nguyen TT, Nguyen VH, Deivasigamani RK, Kharismadewi D, Iwai Y, Shim JJ (2016) Facile synthesis of cobalt oxide/reduced graphene oxide composites for electrochemical capacitor and sensor applications. Solid State Sci 53:71–77
Radhakrishnan S, Krishnamoorthy K, Sekar C, Wilson J, Kim SJ (2014) A highly sensitive electrochemical sensor for nitrite detection based on Fe2O3 nanoparticles decorated reduced graphene oxide nanosheets. Appl Catal B Environ 148:22–28
Rifaya MN, Theivasanthi T, Alagar M (2012) Chemical capping synthesis of nickel oxide nanoparticles and their characterizations studies. Nanosci Nanotechnol 2:134–138
Lang JW, Kong LB, Liu M, Luo YC, Kang L (2010) Co0.56Ni0.44 oxide nanoflake materials and activated carbon for asymmetric supercapacitor. J Electrochem Soc 57(12):A1341–A1346
Zhu M, Zhang X, Zhou Y, Zhuo C, Huang J, Li S (2015) Facile solvothermal synthesis of porous ZnFe2O4 microspheres for capacitive pseudocapacitors. RSC Adv 5:39270–39277
Veerasubramani GK, Krishnamoorthy K, Radhakrishnan S, Kim NJ, Kim SJ (2016) In-situ chemical oxidative polymerization of aniline monomer in the presence of cobalt molybdate for supercapacitor applications. J Indus Eng Chem 36:163–168
Xia XH, JP T, Zhang YQ, Wang XL, CD G, Zhao XB, Fan HJ (2012) High-quality metal oxide core/shell nanowire arrays on conductive substrates for electrochemical energy storage. ACS Nano 6:5531
Zhang F, Yuan CZ, XJ L, Zhang LJ, Che Q, Zhang XG (2012) Facile growth of mesoporous Co3O4 nanowire arrays on Ni foam for high performance electrochemical capacitors. J Power Sources 203:250–256
Mai LQ, Yang F, Zhao YL, Xu X, Xu L, Luo YZ (2011) Hierarchical MnMoO4/CoMoO4 hetero structured nanowires with enhanced supercapacitor performance. Nat Commun 2:381
Liu MC, Kong LB, Lu C, Ma XJ, Li XM, Luo YC, Kang L (2013) Design and synthesis of CoMoO4–NiMoO4·xH2O bundles with improved electrochemical properties for supercapacitors. J Mater Chem A 1:1380
Li X, Walsh FC, Pletcher D (2011) Nickel based electrocatalysts for oxygen evolution in high current density, alkaline water electrolysers. Phys Chem Chem Phys 13:1162–1167
Fleischmann M, Korinek K, Pletcher D (1971) The oxidation of organic compounds at a nickel anode in alkaline solution. J Electroanal Chem 31:39–49
Krishnan SG, Reddy MV, Harilal M, Vidyadharan B, Misnon II, Rahim MHA, Ismail J, Jose R (2015) Characterization of MgCo2O4 as an electrode for high performance supercapacitors. Electrochim Acta 161:312–321
Wang L, Ji H, Wang S, Kong L, Jiang X, Yang G (2013) Preparation of Fe3O4 with high specific surface area and improved capacitance as a supercapacitor. Nano 5:3793–3799
Yan J, Wang Q, Wei T, Fan Z (2014) Recent advances in design and fabrication of electrochemical supercapacitors with high energy densities. Adv Energy Mater 4:1300816
Theerthagiri J, Senthil RA, Buraidah MH, Raghavender M, Madhavan J, Arof AK (2016) Synthesis and characterization of (Ni1-xCox)Se2 based ternary selenides as electrocatalyst for triiodide reduction in dye-sensitized solar cells. J Solid State Chem 238:113–120
Acknowledgements
The authors Mr. KT and Dr.JM are grateful to the authorities of Thiruvalluvar University for their support.
Funding
The authors Dr.PA and Prof. MAG would like to extend their sincere appreciation to the Deanship of Scientific Research at king Saud University for funding this Research group no. RG-1438-087.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thiagarajan, K., Theerthagiri, J., Senthil, R.A. et al. Synthesis of Ni3V2O8@graphene oxide nanocomposite as an efficient electrode material for supercapacitor applications. J Solid State Electrochem 22, 527–536 (2018). https://doi.org/10.1007/s10008-017-3788-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3788-8