Abstract
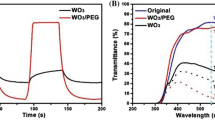
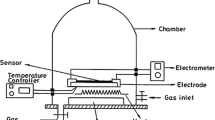
Nanostructured WO3 films were produced by a simple method using ammonium tungstate dissolved in different solvents: ethanol, PEG 300, and a mixture of ethylene glycol with PEG 300. The suspensions were deposited on an FTO substrate by drop casting method and calcined at 500 °C in air atmosphere. The films were characterized by scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), X-ray diffraction (XRD), UV–Vis diffuse reflectance spectroscopy, and photoelectrochemistry measurements. FTO substrates were fully covered by a thin and adherent WO3 film, which presented a nanostructure with particle diameter of 30–80 nm. XRD confirms the monoclinic structure of WO3. Ethanol samples presented higher photocurrent for water oxidation, compared to other solvents. However, these electrodes showed high fragility and the response did not present repeatability. High adhesion was obtained with PEG as solvent (by itself or mixed with ethylene glycol). In addition, WO3 was applied as a photoelectrochemical sensor to detect dopamine under visible light irradiation. The developed sensor showed photosensitivity to dopamine with reproducibility, stability, wide linear range, and low detection limit (0.30 μM).








Similar content being viewed by others
References
Maeda K, Domen K (2010) Photocatalytic water splitting: recent progress and future challenges. J Phys Chem Lett 1:2655–2661
Chen X, Shen S, Guo L, Mao SS (2010) Semiconductor-based photocatalytic hydrogen generation. Chem Rev 110:6503–6570
A a I, DW B (2014) Photochemical splitting of water for hydrogen production by photocatalysis: a review. Sol Energy Mater Sol Cells 128:85–101
Yang J, Wang D, Han H, Li C (2013) Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc Chem Res 46:1900–1909
Paracchino A, Laporte V, Sivula K, Grätzel M, Thimsen E (2011) Highly active oxide photocathode for photoelectrochemical water reduction. Nat Mater 10:456–461
Osterloh FE (2013) Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem Soc Rev 42:2294–2320
Chen HM, Chen CK, Liu R-S, Zhang L, Zhang J, Wilkinson DP (2012) Nano-architecture and material designs for water splitting photoelectrodes. Chem Soc Rev 41:5654–5671
Asai R, Nemoto H, Jia Q, Saito K, Iwase A, Kudo A (2014) A visible light responsive rhodium and antimony-codoped SrTiO3 powdered photocatalyst loaded with an IrO2 cocatalyst for solar water splitting. Chem Commun (Camb) 50:2543–2546
Chen Z, Cummins D, Reinecke BN, Clark E, Sunkara MK, Jaramillo TF (2011) Core-shell MoO 3-MoS 2 nanowires for hydrogen evolution. Nano Lett 11:4168–4175
Chou J-C, Yang M-H, Liao J-W, Lee C-Y, Gan J-Y (2014) Photoexcitation of TiO2 photoanode in water splitting. Mater Chem Phys 143:1417–1422
Nandiyanto ABD, Arutanti O, Ogi T, Iskandar F, Kim TO, Okuyama K (2013) Synthesis of spherical macroporous WO3 particles and their high photocatalytic performance. Chem Eng Sci 101:523–532
Zhao Y, Wei X, Wang Y, Luo F (2014) One-pot twelve tungsten phosphate acid assisted electrochemical synthesis of WO3-decorated graphene sheets for high-efficiency UV-light-driven photocatalysis. Chem Phys Lett 607:34–38
Szilágyi IM, Fórizs B, Rosseler O, et al. (2012) WO3 photocatalysts: influence of structure and composition. J Catal 294:119–127
Hernandez-Uresti DB, Sánchez-Martínez D, Martinez-de la cruz A, Sepúlveda-Guzmán S, Torres-Martínez LM (2014) Characterization and photocatalytic properties of hexagonal and monoclinic WO3 prepared via microwave-assisted hydrothermal synthesis. Ceram Int 40:4767–4775
Luévano-Hipólito E, Martínez-de la Cruz A, Yu QL, Brouwers HJH (2014) Precipitation synthesis of WO3 for NOx removal using PEG as template. Ceram Int 40:12123–12128
Caruso T, Castriota M, Policicchio A, et al. (2014) Thermally induced evolution of sol–gel grown WO3 films on ITO/glass substrates. Appl Surf Sci 297:195–204
Qi C-X, Tan Z, Feng Z-H, Yu L-P (2014) Fabrication of bowl-like porous WO3 film by colloidal crystal template-assisted electrodeposition method. J Mater Sci Mater Electron 25:1553–1558
Shen Y, Yan P, Yang Y, Hu F, Xiao Y, Pan L, Li Z (2015) Hydrothermal synthesis and studies on photochromic properties of Al doped WO3 powder. J Alloys Compd 629:27–31
Houweling ZS, Harks P-PRML, Kuang Y, van der Werf CHM, Geus JW, Schropp REI (2015) Hetero- and homogeneous three-dimensional hierarchical tungsten oxide nanostructures by hot-wire chemical vapor deposition. Thin Solid Films 575:76–83
He Z, Liu Q, Hou H, Gao F, Tang B, Yang W (2015) Tailored electrospinning of WO3 nanobelts as efficient ultraviolet photodetectors with photo-dark current ratios up to 1000. ACS Appl Mater Interfaces 150515092335006 7(20):10878–10885
Santato C, Odziemkowski M, Ulmann M, Augustynski J (2001) Crystallographically oriented mesoporous WO3 films: synthesis, characterization, and applications. J Am Chem Soc 123:10639–10649
Li YJ, Liu ZF, Liang XP, Ya J, Cui T, Liu ZC (2014) Synthesis and electrochromic properties of PEG doped WO 3 film. Mater Technol 29:341–349
Ham DJ, Phuruangrat A, Thongtem S, Lee JS (2010) Hydrothermal synthesis of monoclinic WO3 nanoplates and nanorods used as an electrocatalyst for hydrogen evolution reactions from water. Chem Eng J 165:365–369
Yagi M, Maruyama S, Sone K, Nagai K, Norimatsu T (2008) Preparation and photoelectrocatalytic activity of a nano-structured WO3 platelet film. J Solid State Chem 181:175–182
Yang H, Shang F, Gao L, Han H (2007) Structure, electrochromic and optical properties of WO3 film prepared by dip coating-pyrolysis. Appl Surf Sci 253:5553–5557
Mascaro LH, Pockett A, Mitchels JM, et al. (2014) One-step preparation of the BiVO4 film photoelectrode. J Solid State Electrochem 1–5 19(1):31–35
Tsierkezos NG, Ritter U (2012) Oxidation of dopamine on multi-walled carbon nanotubes. J Solid State Electrochem 16:2217–2226
Tsierkezos NG, Ritter U, Thaha YN, Downing C, Szroeder P (2015) Synthesis, characterization, and electrochemical application of phosphorus-doped multi-walled carbon nanotubes. J Solid State Electrochem 19:891–905
Yang SL, Li G, Yang R, Xia MM, Qu LB (2011) Simultaneous voltammetric detection of dopamine and uric acid in the presence of high concentration of ascorbic acid using multi-walled carbon nanotubes with methylene blue composite film-modified electrode. J Solid State Electrochem 15:1909–1918
Anithaa AC, Lavanya N, Asokan K, Sekar C (2015) WO3 nanoparticles based direct electrochemical dopamine sensor in the presence of ascorbic acid. Electrochim Acta 167:294–302
Ribeiro FWP, Moraes FC, Pereira EC, Marken F, Mascaro LH (2015) New application for the BiVO4 photoanode: a photoelectroanalytical sensor for nitrite. Electrochem Commun 61:1–4
Methods CA (1987) Recommendations for the definition, estimation and use of the detection limit. R Soc Chem 112:199–204
Ti CC, Kumar SA, Chen SM (2009) Electrochemical preparation, characterization, and electrocatalytic studies of Nafion-ruthenium oxide modified glassy carbon electrode. J Solid State Electrochem 13:397–406
Li NB, Ren W, Luo HQ (2008) Simultaneous voltammetric measurement of ascorbic acid and dopamine on poly(caffeic acid)-modified glassy carbon electrode. J Solid State Electrochem 12:693–699
Ardakani MM, Talebi A, Naeimi H, Barzoky MN, Taghavinia N (2009) Fabrication of modified TiO2 nanoparticle carbon paste electrode for simultaneous determination of dopamine, uric acid, and l-cysteine. J Solid State Electrochem 13:1433–1440
Deon M, Caldas EM, da Rosa DS, de Menezes EW, Dias SLP, Pereira MB, Costa TMH, Arenas LT, Benvenutti EV (2015) Mesoporous silica xerogel modified with bridged ionic silsesquioxane used to immobilize copper tetrasulfonated phthalocyanine applied to electrochemical determination of dopamine. J Solid State Electrochem 19(7):2095–2105
Wang G, Jiao H, Liu K, Wu X, Dong Y, Li Z, Zhang C (2014) Electrochemistry Communications Short communication A novel strategy for the construction of photoelectrochemical sensors based on quantum dots and electron acceptor : The case of dopamine detection. Electrochem Commun 41:47–50
Gao P, Ma H, Yang J, Wu D, Zhang Y, Du B, Fan D, Wei Q (2015) Anatase TiO 2 based photoelectrochemical sensor for the sensitive determination of dopamine under visible light irradiation. New J Chem 39:1483–1487
Ma W, Wang L, Zhang N, Han D, Dong X, Niu L (2015) Biomolecule-free, selective detection of o-diphenol and its derivatives with WS2/TiO2-based photoelectrochemical platform. Anal Chem 87:4844–4850
Hao Q, Wang P, Ma X, Su M, Lei J, Ju H (2012) Charge recombination suppression-based photoelectrochemical strategy for detection of dopamine. Electrochem Commun 21:39–41
Yan Y, Liu Q, Du X, Qian J, Mao H, Wang K (2015) Visible light photoelectrochemical sensor for ultrasensitive determination of dopamine based on synergistic effect of graphene quantum dots and TiO2 nanoparticles. Anal Chim Acta 853:258–264
Acknowledgments
The authors would like to thank FAPESP (Process 2014/10757-4 and 2015/ 00231-8), CEPID/FAPESP (Process No. 2013/07296-2), CNPq and CAPES for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alves, S.A., Soares, L.L., Goulart, L.A. et al. Solvent effects on the photoelectrochemical properties of WO3 and its application as dopamine sensor. J Solid State Electrochem 20, 2461–2470 (2016). https://doi.org/10.1007/s10008-016-3144-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3144-4