Abstract
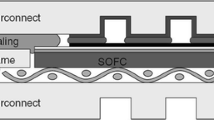
Glasses and glass-ceramics (GCs), in particular alkaline-earth alumino silicate-based compositions, are becoming the most common sealing materials for gas-tight sealing applications in solid oxide fuel cells (SOFCs). The present review aims at reporting the systematic procedures put forward developing a novel concept of diopside-based bilayer GC seal, which contains a rigid GC and a self-healing (SH) GC. The concept behind the bilayer GCs is (i) a small gradient in the coefficient of thermal expansion (CTE) will lead to lower thermal expansion mismatch between the sealing layers and other SOFC components, thus providing enhanced mechanical reliability for the stack; and (ii) cracks produced due to minor thermal stresses in the rigid GC layer can be healed by the SH GC layer due to sufficient amorphous content. In general, at high temperature, highly crystallized glass behaves as a rigid glass. On the other hand, due to low viscosity behavior, partially crystallized glass provides a SH behavior. Various glasses in the field of diopside crystalline materials have been systematically designed by varying the chemical composition of glass to achieve desired combination of functional properties for the rigid and SH GC layers. The glass Sr-0.3 where Sr replaced 30 % of Ca was revealed as a highly reliable rigid GC seal for high-temperature electrochemical applications. On the other hand, SH features have been achieved in 30 % Sr-containing diopside-based glass with Gd2O3 for MgO + SiO2 substitution (denoted as Gd-0.3). These GCs exhibit similar thermal properties and excellent thermal stability along a period of 1000 h, while differing in their amorphous fractions, and revealed excellent thermal stability along a period of 1000 h. The bilayered GC synthesized from Sr-0.3 and Gd-0.3 showed good wetting and bonding ability to the SOFC metallic Crofer22APU components. The results revealed superior performance for the newly proposed bilayer GCs in comparison to single-layer sealants.










Similar content being viewed by others
References
Wachsman ED, Lee KT (2011) Lowering the temperature of solid oxide fuel cells. Science 334:935–939
Wachsman ED, Marlowe CA, Lee KT (2012) Role of solid oxide fuel cells in a balanced energy strategy. Energy Environ Sci 5:5498–5509
Minh NK, Takahashi T (1995) Science and technology of ceramic fuel cells. Elsevier, Amsterdam
Larminie J, Dicks A (2003) Fuel cell systems explained, 2nd edn. Wiley, West Sussex
Milhans J, Ahzi S, Garmestani H, Khaleel MA, Sun X, Koeppel BJ (2009) Modeling of the effective elastic and thermal properties of glass-ceramic solid oxide fuel cell seal materials. Mater Des 30:1667–1673
Haile S, Dane A, Boysen D, Chisholm C, Merle R (2001) Solid acids as fuel cell electrolytes. Nature 410:910–914
Zhong C-J, Luo J, Njoki PN, Mott D, Wanjala B, Loukrakpam R, Lim S, Wang L, Fang B, Xu Z (2008) Fuel cell technology: nano-engineered multimetallic catalysts. Energy Environ Sci 1:454–466
de Bruijn F (2005) The current status of fuel cell technology for mobile and stationary applications. Green Chem 7:132–150
Kendrick E, Slater P (2012) Battery and solid oxide fuel cell materials. Annu Rep Sect A Inorg Chem 108:424–448
Ormerod RM (2003) Solid oxide fuel cells. Chem Soc Rev 32:17–28
Zhao Y, Xia C, Jia L, Wang Z, Li H, Yu J, Li Y (2013) Recent progress on solid oxide fuel cell: lowering temperature and utilizing non-hydrogen fuels. Int J Hydrog Energy 38:16498–16517
Fergus JW (2005) Sealants for solid oxide fuel cells. J Power Sources 147:46–57
Tulyaganov DU, Reddy AA, Kharton VV, Ferreira JMF (2013) Aluminosilicate-based sealants for SOFCs and other electrochemical applications—a brief review. J Power Sources 242:486–502
Batfalsky P, Haanappel VAC, Malzbender J, Menzler NH, Shemet V, Vinke IC, Steinbrech RW (2006) Chemical interaction between glass–ceramic sealants and interconnect steels in SOFC stacks. J Power Sources 155:128–137
Liu WN, Sun X, Khaleel MA (2011) Study of geometric stability and structural integrity of self-healing glass seal system used in solid oxide fuel cells. J Power Sources 196:1750–1761
Govindaraju N, Liu WN, Sun X, Singh P, Singh RN (2009) A modeling study on the thermomechanical behavior of glass-ceramic and self-healing glass seals at elevated temperatures. J Power Sources 190:476–484
Singh RN (2007) Sealing technology for solid oxide fuel cells (SOFC). Int J Appl Ceram Technol 4:134–144
Story C, Lu K, Reynoldsjr W, Brown D (2008) Shape memory alloy/glass composite seal for solid oxide electrolyzer and fuel cells. Int J Hydrog Energy 33:3970–3975
SØrensen BF, Sarraute S, JØrgensen O, Horsewell A (1998) Thermally induced delamination of multilayers. Acta Mater 46:2603–2615
Coillot D, Méar FO, Podor R, Montagne L (2010) Autonomic self-repairing glassy materials. Adv Funct Mater 20:4371–4374
Reddy AA, Tulyaganov DU, Kapoor S, Goel A, Pascual MJ, Kharton VV, Ferreira JMF (2012) Study of melilite based glasses and glass-ceramics nucleated by Bi2O3 for functional applications. RSC Adv 2:10955–10967
Reddy AA, Tulyaganov DU, Goel A, Kapoor S, Pascual MJ, Ferreira JMF (2013) Sintering and devitrification of glass-powder compacts in the akermanite–gehlenite system. J Mater Sci 48:4128–4136
Reddy AA, Tulyaganov DU, Goel A, Sardo M, Wiper PV, Pascual MJ, Kharton VV, Kolotygin VA, Tsipis EV, Mafra L, Ferreira JMF (2013) Melilite glass–ceramic sealants for solid oxide fuel cells: effects of ZrO2 additions assessed by microscopy, diffraction and solid-state NMR. J Mater Chem A 1:6471
Smeacetto F, Chrysanthou A, Salvo M, Zhang Z, Ferraris M (2009) Performance and testing of glass-ceramic sealant used to join anode-supported-electrolyte to Crofer22APU in planar solid oxide fuel cells. J Power Sources 190:402–407
Lin C-K, Lin K-L, Yeh J-H, Wu S-H, Lee R-Y (2014) Creep rupture of the joint of a solid oxide fuel cell glass–ceramic sealant with metallic interconnect. J Power Sources 245:787–795
Ito Y (2013) Chapter 10.2—heat-resistant coating technology for gas turbines. In: Somiya S (ed) Handbook of advanced ceramics, 2nd edn. Academic, Oxford, pp 789–806
Itoh Y, Kameda T, Okamura T, Nagata K (1999) Sintering behavior of zirconia thermal barrier coating under gradient temperature. J Soc Mater Sci Jpn 48:740–745
Mahapatra MK, Lu K (2010) Seal glass for solid oxide fuel cells. J Power Sources 195:7129–7139
Mahapatra MK, Lu K (2010) Glass-based seals for solid oxide fuel and electrolyzer cells—a review. Mater Sci Eng R 67:65–85
Nascimento ML, Ferreira EB, Zanotto ED (2004) Kinetics and mechanisms of crystal growth and diffusion in a glass-forming liquid. J Chem Phys 121:8924–8928
Hayashi S, Okada K, Ōtsuka N (1990) Sintering behaviour of diopside, CaMgSi2O6, from various powder preparation methods. J Mater Sci Lett 9:382–385
Finger LW, Ohashi Y (1976) The thermal expansion of diopside to 800°C and a refinement of the crystal structure at 700°C. Am Mineral 61:303–310
Alekseev YI, Galanov YI (1990) Electrical conductivity of diopside ceramics. Glass Ceram 47:24–26
Cameron M, Papike JJ (1981) Structural and chemical variations in pyroxenes. Am Mineral 66:1–50
Benna P (1982) Ca–Sr substitution in clinopyroxenes along the join CaMgSi2O6–SrMgSi2O6. TMPM Tschermaks Petrogr Mitt 30:37–46
Newton RC, Charlu TV, Anderson PAM, Kleppa OJ (1979) Thermochemistry of synthetic clinopyroxenes on the join CaMgSi2O6-Mg2Si2O6. Geochim Cosmochim Acta 43:55–60
Benna P, Chiari G, Bruno E (1987) Structural modifications in clinopyroxene solid solutions: the Ca-Mg and Ca-Sr substitutions in the diopside structure. Mineral Petrol 36:71–84
Raudsepp M, Hawthorne FC, Turnock AC (1990) Crystal chemistry of synthetic pyroxenes on the join CaNiSi2O6-CaMgSi2O6 (diopside): a Rietveld structure refinement study. Am Mineral 75:1274–1281
Gottschalk M, Najorka J, Andrut M (1998) Structural and compositional characterization of synthetic (Ca, Sr)-tremolite and (Ca, Sr)-diopside solid solutions. Phys Chem Miner 25:415–428
Najorka J, Gottschalk M, Franz G, Heinrich W (1999) Ca-Sr distribution among amphibole, clinopyroxene, and chloride-bearing solutions. Am Mineral 84:596–606
Bruno E, Carbonin S, Molin G (1982) Crystal structures of Ca-rich clinopyroxenes on the CaMgSi2O6-Mg2Si2O6 join. TMPM Tschermaks Petrogr Mitt 29:223–240
Ohashi Y, Burnham CW, Finger LW (1975) The effect of Ca-Fe substitution on the clinopyroxene crystal structure. Am Mineral 60:423–434
Huang E, Chen CH, Huang T, Lin EH, Xu J-A (2000) Raman spectroscopic characteristics of Mg-Fe-Ca pyroxenes. Am Mineral 85:473–479
Tribaudino M, Nestola F (2002) Average and local structure in P21/c clinopyroxenes along the join diopside-enstatite (CaMgSi2O6-Mg2Si2O6). Eur J Miner 14:549–555
Wu C, Ramaswamy Y, Zreiqat H (2010) Porous diopside (CaMgSi2O6) scaffold: a promising bioactive material for bone tissue engineering. Acta Biomater 6:2237–2245
Goel A, Reddy AA, Pascual MJ, Gremillard L, Malchere A, Ferreira JMF (2012) Sintering behavior of lanthanide-containing glass-ceramic sealants for solid oxide fuel cells. J Mater Chem 22:10042
Reddy AA, Tulyaganov DU, Goel A, Pascual MJ, Kharton VV, Tsipis EV, Ferreira JMF (2012) Diopside–Mg orthosilicate and diopside–Ba disilicate glass–ceramics for sealing applications in SOFC: sintering and chemical interactions studies. Int J Hydrog Energy 37:12528–12539
Abo-Mosallam HA, Hill RG, Karpukhina N, Law RV (2010) MAS-NMR studies of glasses and glass-ceramics based on a clinopyroxene–fluorapatite system. J Mater Chem 20:790
Fernandes HR, Tulyaganov DU, Ribeiro MJ, Ferreira JMF (2013) Apatite crystallization from glasses in the Ca5(PO4)3F–CaAl2Si2O8–CaMgSi2O6–NaAlSi3O8 system. J Non-Cryst Solids 363:32–38
Goel A, Rajagopal RR, Ferreira JMF (2011) Influence of strontium on structure, sintering and biodegradation behaviour of CaO–MgO–SrO–SiO2–P2O5–CaF2 glasses. Acta Biomater 7:4071–4080
Zhang J, Zhou Y, Yue Z (2013) Low-temperature sintering and microwave dielectric properties of LiF-doped CaMg1−xZnxSi2O6 ceramics. Ceram Inter 39:2051–2058
Lecointre A, Bessière A, Priolkar KR, Gourier D, Wallez G, Viana B (2013) Role of manganese in red long-lasting phosphorescence of manganese-doped diopside for in vivo imaging. Mater Res Bull 48:1898–1905
Ghorbanian L, Emadi R, Razavi SM, Shin H, Teimouri A (2013) Fabrication and characterization of novel diopside/silk fibroin nanocomposite scaffolds for potential application in maxillofacial bone regeneration. Int J Biol Macromol 58:275–280
Maldiney T, Lecointre A, Viana B, Bessière A, Bessodes M, Gourier D, Richard C, Scherman D (2011) Controlling electron trap depth to enhance optical properties of persistent luminescence nanoparticles for in vivo imaging. J Am Chem Soc 133:11810–11815
Goel A, Tulyaganov DU, Ferrari AM, Shaaban ER, Prange A, Bondioli F, Ferreira JMF (2010) Structure, sintering, and crystallization kinetics of alkaline-earth aluminosilicate glass-ceramic sealants for solid oxide fuel cells. J Am Ceram Soc 93:830–837
Goel A, Tulyaganov DU, Kharton VV, Yaremchenko AA, Ferreira JMF (2010) Electrical behavior of aluminosilicate glass-ceramic sealants and their interaction with metallic solid oxide fuel cell interconnects. J Power Sources 195:522–526
Kerstan M, Rüssel C (2011) Barium silicates as high thermal expansion seals for solid oxide fuel cells studied by high-temperature X-ray diffraction (HT-XRD). J Power Sources 196:7578–7584
Ojha PK, Chongdar TK, Gokhale NM, Kulkarni AR (2013) Accelerated devitrification of a strontiumlanthanumaluminoborosilicate based intermediate temperature solid oxide fuel cell glass sealant and its effect on thermophysical behaviour of the glass ceramics. J Power Sources 221:28–34
Lin C-K, Lin K-L, Yeh J-H, Shiu W-H, Liu C-K, Lee R-Y (2013) Aging effects on high-temperature creep properties of a solid oxide fuel cell glass-ceramic sealant. J Power Sources 241:12–19
Pauling L (1929) The principles determining the structure of complex ionic crystals. J Am Chem Soc 51:1010–1026
Phillips AH (1932) Isomorphous substitution of elements in minerals. Am Mineral 17:85–93
Park B, Li H, Corrales LR (2002) Molecular dynamics simulation of La2O3–Na2O–SiO2 glasses. I. The structural role of La3+ cations. J Non-Cryst Solids 297:220–238
Corrales LR, Park B (2002) Molecular dynamics simulation of La2O3–Na2O–SiO2 glasses. III. The driving forces of clustering. J Non-Cryst Solids 311:118–129
Park B, Corrales LR (2002) Molecular dynamics simulation of La2O3–Na2O–SiO2 glasses. II. The clustering of La3+ cations. J Non-Cryst Solids 311:107–117
Schaller T, Stebbins JF, Wilding MC (1999) Cation clustering and formation of free oxide ions in sodium and potassium lanthanum silicate glasses: nuclear magnetic resonance and Raman spectroscopic findings. J Non-Cryst Solids 243:146–157
Zielinski RA, Frey FA (1974) An experimental study of the partitioning of a rare earth element (Gd) in the system diopside—aqueous vapour. Geochim Cosmochim Acta 38:545–565
Catlow CRA, Thomas JM, Parker SC, Jefferson DA (1982) Simulating silicate structures and the structural chemistry of pyroxenoids. Nature 295:658–662
Li L, Strachan DM, Li H, Davis LL, Qian M (2000) Crystallization of gadolinium- and lanthanum-containing phases from sodium alumino-borosilicate glasses. J Non-Cryst Solids 272:46–56
Quintas A, Caurant D, Majérus O, Charpentier T, Dussossoy JL (2009) Effect of compositional variations on charge compensation of AlO4 and BO4 entities and on crystallization tendency of a rare-earth-rich aluminoborosilicate glass. Mater Res Bull 44:1895–1898
Felsche J (1972) Rare earth silicates with the apatite structure. J Solid State Chem 5:266–275
Quintas A, Caurant D, Majérus O, Dussossoy J-L (2008) Effect of changing the rare earth cation type on the structure and crystallisation behaviour of an aluminoborosilicate glass. Phy Chem Glasses Eur J Glass Sci Tech B 49:192–197
Goel A, Tulyaganov DU, Kharton VV, Yaremchenko AA, Eriksson S, Ferreira JMF (2009) Optimization of La2O3-containing diopside based glass-ceramic sealants for fuel cell applications. J Power Sources 189:1032–1043
Otto K, Wisniewski W, Rüssel C (2013) Growth mechanisms of surface crystallized diopside. CrystEngComm 15:6381
Tang XP, Geyer U, Busch R, Johnson WL, Wu Y (1999) Diffusion mechanisms in metallic supercooled liquids and glasses. Nature 402:160–162
Ray NH (1974) Composition–property relationships in inorganic oxide glasses. J Non-Cryst Solids 15:423–434
Murdoch JB, Stebbins JF, Carmichael ISE (1985) High-resolution 29Si NMR study of silicate and aluminosilicate glasses: the effect of network-modifying cations. Am Mineral 70:332–343
Rawson H (1967) Inorganic glass-forming systems. Academic, London
Branda F, Costantini A, Luciani G, Laudisio G (2001) The role of trivalent element oxides in Cao (Na2O)–M2O3–SiO2 glasses from Tg. J Therm Anal Calorim 64:1017–1024
Buesser B, Gröhn AJ, Pratsinis SE (2011) Sintering rate and mechanism of TiO2 nanoparticles by molecular dynamics. J Phys Chem C 115:11030–11035
Xu J, Sakanoi R, Higuchi Y, Ozawa N, Sato K, Hashida T, Kubo M (2013) Molecular dynamics simulation of Ni nanoparticles sintering process in Ni/YSZ multi-nanoparticle system. J Phys Chem C 117:9663–9672
Pascual MJ, Durán A, Pascual L (2002) Sintering process of glasses in the system Na2O–B2O3–SiO2. J Non-Cryst Solids 306:58–69
Boccaccini AR (1993) Anisotropic densification during sintering of glass powder compacts. J Mater Sci Lett 12:943–945
Lara C, Pascual M, Prado M, Duran A (2004) Sintering of glasses in the system RO–Al2O3–BaO–SiO2 (R = Ca, Mg, Zn) studied by hot-stage microscopy. Solid State Ionics 170:201–208
Lara C, Pascual MJ, Durán A (2004) Glass-forming ability, sinterability and thermal properties in the systems RO–BaO–SiO2 (R = Mg, Zn). J Non-Cryst Solids 348:149–155
Garza-Garcia M, López-cuevas J, Gutiérrez-chavarría CA, Rendón-Angeles JC, Valle-Fuentes JF (2007) Study of a mixed alkaline–earth effect on some properties of glasses of the CaO-MgO-Al2O3-SiO2 system. Bull Span Soc Ceram Glass 46:153–162
Isard JO (1969) The mixed alkali effect in glass. J Non-Cryst Solids 1:235–261
Branda F, Buri A, Caferra D, Marotta A (1983) The effect of mixing of network-modifiers on the transformation temperature of silicate glasses. J Non-Cryst Solids 54:193–198
Reddy AA, Tulyaganov DU, Pascual MJ, Kharton VV, Tsipis EV, Kolotygin VA, Ferreira JMF (2013) Diopside–Ba disilicate glass–ceramic sealants for SOFCs: enhanced adhesion and thermal stability by Sr for Ca substitution. Int J Hydrog Energy 38:3073–3086
Nakamura E, Kushiro I (1998) Trace element diffusion in jadeite and diopside melts at high pressures and its geochemical implication. Geochim Cosmochim Acta 62:3151–3160
Stephens EV, Vetrano JS, Koeppel BJ, Chou Y, Sun X, Khaleel MA (2009) Experimental characterization of glass–ceramic seal properties and their constitutive implementation in solid oxide fuel cell stack models. J Power Sources 193:625–631
Chang H-T, Lin C-K, Liu C-K, Wu S-H (2011) High-temperature mechanical properties of a solid oxide fuel cell glass sealant in sintered forms. J Power Sources 196:3583–3591
Milhans J, Li D, Khaleel M, Sun X, Garmestani H (2010) Statistical continuum mechanics analysis of effective elastic properties in solid oxide fuel cell glass–ceramic seal material. J Power Sources 195:5726–5730
Bansal NP, Gamble EA (2005) Crystallization kinetics of a solid oxide fuel cell seal glass by differential thermal analysis. J Power Sources 147:107–115
Lennard-Jones JE, Dent BM (1928) Cohesion at a crystal surface. Trans Faraday Soc 24:92–108
Magi M, Lippmaa E, Samoson A, Engelhardt G, Grimmer AR (1984) Solid-state high-resolution silicon-29 chemical shifts in silicates. J Phys Chem 88:1518–1522
Stebbins JF, Murdoch JB, Schneider E, Carmichael ISE, Pines A (1985) A high-temperature high-resolution NMR study of 23Na, 27Al and 29Si in molten silicates. Nature 314:250–252
Muller E (1992) Influence of cation coordination on nucleation in silicate glasses. Z Krist 200:287–294
De Jong BHWS, Schramm CM, Parziale VE (1984) Silicon-29 magic angle spinning NMR study on local silicon environments in amorphous and crystalline lithium silicates. J Am Chem Soc 106:4396–4402
Nascimento M, Zanotto E (2006) Mechanisms and dynamics of crystal growth, viscous flow, and self-diffusion in silica glass. Phys Rev B 73:024209
Cormier L, Calas G, Cuello GJ (2010) Structural study of Ca–Mg and K–Mg mixing in silicate glasses by neutron diffraction. J Non-Cryst Solids 356:2327–2331
Lee S, Mysen B, Cody G (2003) Chemical order in mixed-cation silicate glasses and melts. Phys Rev B 68:214206
Reddy AA, Tulyaganov DU, Pascual MJ, Kharton VV, Tsipis EV, Kolotygin VA, Ferreira JMF (2013) SrO-containing diopside glass-ceramic sealants for solid oxide fuel cells: mechanical reliability and thermal shock resistance. Fuel Cells 13:689–694
Sohn S-B, Choi S-Y, Kim G-H, Song H-S, Kim G-D (2004) Suitable glass-ceramic sealant for planar solid-oxide fuel cells. J Am Ceram Soc 87:254–260
Reddy AA, Goel A, Tulyaganov DU, Sardo M, Mafra L, Pascual MJ, Kharton VV, Tsipis EV, Kolotygin VA, Ferreira JMF (2014) Thermal and mechanical stability of lanthanide-containing glass–ceramic sealants for solid oxide fuel cells. J Mater Chem A 2:1834
Shimizu F, Tokunaga H, Saito N, Nakashima K (2006) Viscosity and surface tension measurements of RE2O3–MgO–SiO2 (RE = Y, Gd, Nd and La) melts. ISIJ Int 46:388–393
Chang H-T, Lin C-K, Liu C-K (2010) Effects of crystallization on the high-temperature mechanical properties of a glass sealant for solid oxide fuel cell. J Power Sources 195:3159–3165
Weibull W (1951) A statistical distribution function of wide applicability. J Appl Mech 18:293–297
Kingery DRUWD, Bowen HKNY (1976) Introduction to ceramics, 2nd edn. Wiley, New York
Goel A, Tulyaganov DU, Kharton VV, Yaremchenko AA, Ferreira JMF (2008) The effect of Cr2O3 addition on crystallization and properties of La2O3-containing diopside glass-ceramics. Acta Mater 56:3065–3076
Lara C, Pascual MJ, Keding R, Durán A (2006) Electrical behaviour of glass–ceramics in the systems RO–BaO–SiO2 (R = Mg, Zn) for sealing SOFCs. J Power Sources 157:377–384
Kharton V, Marques F, Atkinson A (2004) Transport properties of solid oxide electrolyte ceramics: a brief review. Solid State Ionics 174:135–149
Jr M (2004) Mechanical and thermal stresses in multilayered materials. J Appl Phys 95:1780
Acknowledgments
This study was partially supported by the CICECO, University of Aveiro, by the JECS-trust frontiers of research (201242-2), and FCT, Portugal (PTDC/CTM–CER/114209/2009). A.A. Reddy thanks FCT for the doctoral grant (SFRH/BD/89915/2012). V.V. Kharton acknowledges also the financial support of the Ministry of Education and Science of the Russian Federation (agreement 14.B25.31.0018) and the Russian Foundation for Basic Research (grant 13-03-12409). Important experimental contributions, partly reviewed in this work, and helpful discussions made by A. Goel and M.J. Pascual are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Reddy, A.A., Tulyaganov, D.U., Kharton, V.V. et al. Development of bilayer glass-ceramic SOFC sealants via optimizing the chemical composition of glasses—a review. J Solid State Electrochem 19, 2899–2916 (2015). https://doi.org/10.1007/s10008-015-2925-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-015-2925-5