Abstract
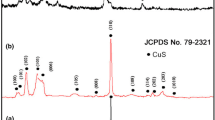
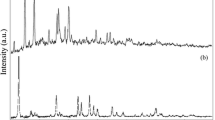
A novel method for the electrochemical synthesis of covellite (CuS) nanoparticles (NPs) in aqueous phase was developed. In this experiment, thioglycerol (TG) is used as the catalyst for the hydrolysis of sodium thiosulfate, the sulfur source for the synthesis of CuS. Cu foil, which acts as the sacrificing anode, is oxidized to Cu2+ by applying a potential of 0.5 V while OH- was produced on the cathode surface at the same time. The production of OH- facilitates the reaction between Cu2+ and thiosulfate under the catalysis of TG. The evolution of hydrogen bubbles effectively prevents the deposition of copper sulfide on the cathode. Copper sulfide sols of “golden-brown”, and “dark-green” forms can be obtained by varying the concentration of TG. The “golden-brown” copper sulfide sols are also observed to convert to the green form with time, and the rate of this conversion process is faster at higher temperatures. X-ray diffraction (XRD) and chemical analysis indicate that the “dark-green” form of product is pure hexagonal phase CuS. The obtained CuS NPs were covered by a layer of TG as suggested by Fourier transform infrared (FTIR) data. The size and morphology of the particles are studied by transmission electron microscope (TEM).






Similar content being viewed by others
References
Zhang YH, Chen F, Zhuang JH, Tang Y, Wang DJ, Wang YJ, Dong AG, Ren N (2002) Chem Commun 23:2814
Guo DJ, Li HL (2004) Electrochem Commun 6:999
Mazur M (2004) Electrochem Commun 6:400
Reetz MT, Helbig W (1994) J Am Chem Soc 116:7401
Rodrigues-Sanchez L, Blanco MC, Lopez-Quintela MA (2000) J Phys Chem B 104:9683
Yu YY, Chang SS, Lee CL, Wang CRC (1997) J Phys Chem B 101:6661
Mohamed MB, Ismail KZ, Link S, El-Sayed MA (1998) J Phys Chem B 102:9370
Liu YC, Li HL (2004) Electrochem Commun 6:1163
Reisse J, Francois H, Vandercammen J, Fabre O, Mesmaeker AKD, Maerschalk C, Delplancke JL (1994) Electrochim. Acta 39:37
Delplancke JL, Dille J, Reisse J, Long GJ, Mohan A, Grandjean F (2000) Chem Mater 12:946
Shen LM, Yao JL, Gu RA (2007) Acta Chimica Sinica 65:203
Ma HY, Yin BS, Wang SY, Jiao YL, Pan W, Huang SX, Chen SH, Meng FJ (2004) Chem Phys Chem 5:68
Yin BS, Ma HY, Wang SY, Chen SH (2003) J Phys Chem B 107:8898
Huang CJ, Chiu PH, Wang YH, Chen KL, Linn JJ, Yang CF (2006) J Electrochem Soc 153:D193
Franger S, Berthet P, Berthon J (2004) J Solid State Eletrochem 8:218
Yang YJ, He LY, Zhang QF (2005) Electrochem Comm 7:361
Grozdanov I, Najdoski M (1995) J Solid State Chem 14:469
Nascu C, Pop I, Ionescu V, Indrea E, Bratu I (1997) Mater Lett 32:73
Hermann AM, Fabick L (1983) J Cryst Growth 61:658
Wang M, Sun L, Fu X, Liao C, Yan C (2000) Solid State Commun 115:493
Hu J, Deng B, Zhang W, Tang K, Qian Y (2001) Int J Inorg Mater 3:639
Takase K, Koyano M, Shimizu T, Makihara K, Takahashi Y, Takano Y, Sekizawa K (2002) Solid State Commun 123:531
Qiao Z, Xie Y, Xu J, Zhu Y, Qian Y (1999) J Colloid Interface Sci 214:459
Zhang Y, Qiao Z, Chen X (2002) J Solid State Chem 167:249
Wang H, Zhang J, Zhao X, Xu S, Zhu J (2002) Mater Lett 55:253
Lu J, Zhao Y, Chen N, Xie Y (2003) Chem Lett 32:30
Wang C, Tang K, Yang Q, Bin H, Shen G, Qian Y (2001) Chem Lett 494
Zhang P, Gao L (2003) J Mater Chem 13:2007
Wu CY, Yu SH, Chen SF, Liu GN, Liu BH (2006) J Mater Chem 16:3326
Qin AM, Fang YP, Ou HD, Liu HQ, Su CY (2005) Cryst Growth Design 5:855
Zhang HT, Wu G, Chen XH (2006) Mater Chem Phys 98:298
Ji HM, Cao JM, Feng J, Chang X, Ma XJ, Liu JS, Zheng MB (2005) Mater Lett 59:3169
Tan CH, Zhu YL, Lu R, Xue PC, Bao CY, Liu XL, Fei ZP, Zhao YY (2005) Mater Chem Phys 91:44
Ni YH, Wang F, Liu HJ, Liang YY, Yin G, Hong JM, Ma X, Xu Z (2003) Inorg Chem Comm 6:1406
Yang YJ, Xiang JW (2005) Appl Phys A 81:1351
Ewen JS, Franz G, Brett AS, Thomas WH (1991) Langmuir 7:2917
Zhang P, Gao L (2003) J Mater Chem 13:2007
Dixit SG, Mahadeshwar AR, Haram SK (1998) Coll Surf A 133:69
Haram SK, Mahadeshwar AR, Dixit SG (1996) J Phys Chem 100:5868
Chen SW, Templeton AC, Murray RW (2000) Langmuir 16:3543
Chen SW, Murray RW. J Phys Chem B 103:9996
Acknowledgment
Financial support from the National Nature Science Foundation of China (Nos. 30370397 and 60571042) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Y.J., Hu, S. A facile electrochemical synthesis of covellite nanomaterials at room temperature. J Solid State Electrochem 12, 1405–1410 (2008). https://doi.org/10.1007/s10008-007-0481-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-007-0481-3