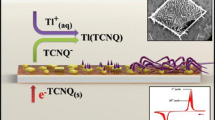
Abstract
In situ atomic force microscopy (AFM) allows images from the upper face and sides of TCNQ crystals to be monitored during the course of the electrochemical solid–solid state conversion of 50 × 50 μm2 three-dimensional drop cast crystals of TCNQ to CuTCNQ or M[TCNQ]2(H2O)2 (M = Co, Ni). Ex situ images obtained by scanning electron microscopy (SEM) also allow the bottom face of the TCNQ crystals, in contact with the indium tin oxide or gold electrode surface and aqueous metal electrolyte solution, to be examined. Results show that by carefully controlling the reaction conditions, nearly mono-dispersed, rod-like phase I CuTCNQ or M[TCNQ]2(H2O)2 can be achieved on all faces. However, CuTCNQ has two different phases, and the transformation of rod-like phase 1 to rhombic-like phase 2 achieved under conditions of cyclic voltammetry was monitored in situ by AFM. The similarity of in situ AFM results with ex situ SEM studies accomplished previously implies that the morphology of the samples remains unchanged when the solvent environment is removed. In the process of crystal transformation, the triple phase solid∣electrode∣electrolyte junction is confirmed to be the initial nucleation site. Raman spectra and AFM images suggest that 100% interconversion is not always achieved, even after extended electrolysis of large 50 × 50 μm2 TCNQ crystals.









Similar content being viewed by others
References
Acker DS et al (1960) J Am Chem Soc 82:6408–6409
Krysinski P, Tien HT (1988) Bioelectrochem and Bioenerg 19:227–233
Freund MS, Brajtertoth A, Ward MD (1990) J Electroanal Chem 289:127–141
Nakajima K, Kageshima M, Ara N, Yoshimura M, Kawazu A (1993) Appl Phys Lett 62:1892–1894
Bond AM, Fletcher S, Marken F, Shaw SJ, Symons PG (1996) J Chem Soc-Faraday Trans 92:3925–3933
Chambers JQ, Scaboo K, Evans CD (1996) J Electrochem Soc 143:3039–3045
Bartlett PN, Tong XQ (1997) J Phys Chem B 101:8540–8549
Bond AM, Fiedler DA (1997) J Electrochem Soc 144:1566–1574
Bond AM, Fletcher S, Symons PG (1998) Analyst 123:1891–1904
Oyama M et al (1998) J Phys Chem B 102:6588–6595
Bellec V et al (2001) Electrochem Comm 3:483–488
Fraxedas J, Langer J, Diez I, Sanz F (2006) J Low Temp Phys 142:121–126
Gomez L, Rodriguez-Amaro R (2006) Langmuir 22:7431–7436
Liu YL et al (2006) J Am Chem Soc 128:12917–12922
O’Mullane AP, Neufeld AK, Bond AM (2005) Anal Chem 77:5447–5452
Neufeld AK, O’Mullane AP, Bond AM (2005) J Am Chem Soc 127:13846–13853
Harris AR, Neufeld AK, O’Mullane AP, Bond AM, Morrison RJS (2005) J Electrochem Soc 152:C577–C583
Cao GY et al (2005) Micron 36:267–270
Zhang Q, Kong LZ, Zhang QJ, Wang WJ, Hua ZY (2004) Solid State Commun 130:799–802
Liu SG, Liu YQ, Wu PJ, Zhu DB (1996) Chem Mater 8:2779–2787
Heintz RA et al (1999) Inorg Chem 38:144–156
Liu YL et al (2005) Adv Mater 17:2953–2957
Potember RS, Poehler TO, Cowan DO, Bloch AN (1981) Bull Amer Phys Soc 26:309
Neufeld AK, Madsen I, Bond AM, Hogan CF (2003) Chem Mater 15:3573–3585
Nafady A, O’Mullane AP, Bond AM, Neufeld AK (2006) Chem Mater 18:4375–4384
Hoagland JJ, Wang XD, Hipps KW (1993) Chem Mater 5:54–60
Wan LJ, Itaya K (2001) Chin Sci Bull 46:377–380
Magonov SN, Bar G, Cantow HJ, Ren J, Whangbo MH (1994) Synth Met 62:159–167
Higo M, Lu X, Mazur U, Hipps KW (2001) Thin Solid Films 384:90–101
Suarez MF, Bond AM, Compton RG (1999) J Solid State Electrochem 4:24–33
Bond AM (2002) Broadening electrochemical horizons: principles and illustration of voltammetric and related techniques, Chapter 5. Oxford University Press, Oxford
Nafady A, Bond AM (2007) Inorg Chem 46:4128–4137
Bak E, Donten M, Stojek Z, Scholz F (2007) Electrochem Comm 9:386–392
Deng Y et al (2005) J Phys Chem B 109:14043–14051
Hasse U, Scholz F (2001) Electrochem Comm 3:429–434
Hermes M, Scholz F (2000) Electrochem Comm 2:845–850
Suchanski MR, Vanduyne RP (1976) J Am Chem Soc 98:250–252
Hu ZP et al (1995) J Mol Struct 356:163–168
Makowski M, Pawlikowski MT (2006) Int J Quantum Chem 106:1736–1748
Gucciardi PG, Trusso S, Vasi C, Patane S, Allegrini M (2002) Phys Chem Chem Phys 4:2747–2753
Acknowledgment
The Australian Research Council is gratefully acknowledged for financial support of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qu, X., Nafady, A., Mechler, A. et al. AFM study of morphological changes associated with electrochemical solid–solid transformation of three-dimensional crystals of TCNQ to metal derivatives (metal = Cu, Co, Ni; TCNQ = tetracyanoquinodimethane). J Solid State Electrochem 12, 739–746 (2008). https://doi.org/10.1007/s10008-007-0423-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-007-0423-0