Abstract
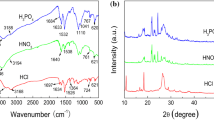
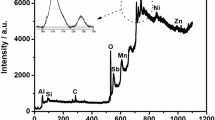
The polypyrrole coating was prepared from phosphoric acid aqueous solution containing the hetero-polyanion \({\text{PMo}}_{{{\text{12}}}} {\text{O}}^{{{\text{3 - }}}}_{{{\text{40}}}} \) and pyrrole monomer to make new coating for corrosion prevention of carbon steels. The coating thus formed in the phosphate acid solution was doped by \({\text{PMo}}_{{{\text{12}}}} {\text{O}}^{{{\text{3 - }}}}_{{{\text{40}}}} \) and by \({\text{PO}}^{{{\text{3 - }}}}_{{\text{4}}} \). The coating was flexible enough to cover the steel homogeneously without cracks, although many cracks were observed on the coating formed in a neutral aqueous solution of Na2MoO2. The 5.1-μm-thick polypyrrole coating makes the steel maintain the passive state for 48 h in neutral 3.5 wt% NaCl solution at pH 5.3 and for 80 h in acidic 3.5 wt% NaCl solution at pH 1.9. The coating decreased the corrosion rate of the steel by 1/200 in the neutral NaCl solution and by 1/340 in the acidic NaCl solution, if compared with the rate of the bare steel. The dissolution current of the steel during the immersion remained at the level of the typical passive current in the respective solutions.









Similar content being viewed by others
References
DeBerry DW(1985) J Electrochem Soc 132:1022
Malik MA, Galkowski MT, Bala H, Grzybowska B, Kulesza PJ (1999) Electrochim Acta 44:2157
Gasparac R, Martin C-R (2001) J Electrochem Soc 148:B138
Gasparac R, Martin C-R (2002) J Electrochem Soc 149:B409
Su W, Iroh JO (1997) Electrochim Acta 42:2685
Kraljic M, Mandic Z, Duic Lj (2003) 45:181
Cheung KM, Bloor D, Stevens GC (1988) Polymer 29:1709
Schirmeisen M, Beck F (1989) J Appl Electrochem 19:401
Fenelson AM, Breslin CB (2002) Electrochim Acta 47:4467
Bazzaoui M, Martin L, Bazzoui EA, Martin JI (2002) J Electroanal Chem 537:47
Tallman DE, Vang C, Wallace GG, Bierwagen GP (2002) J Electrochem Soc 149:C173
Deng Z, Smyrl WH, White H (1989) J Electrochem Soc 136:2152
Fenelon AM, Breslin CB (2003) Corros Sci 45:2837
Wessling B (1994) Adv Mater 6:1994
Lu W-K, Elsenbaumer RL, Wessling B (1995) Synth Met 71:2163
Beck F, Michaelis R, Schloten F, Zinger B (1994) Electrochim Acta 39:229
Krstajic NV, Grgur BN, Sovanovic SM,Vojnovic MV (1997) Electrochim Acta 42:1685
Su W, Iroh JO (1999) Electrochim Acta 44:2173
Su W, Iroh JO (1999) Electrochim Acta 44:3321
Su W, Iroh JO (2000) Electrochim Acta 46:1
Nguyen H, Li T, Gacia B, Deslouis C, Xuan L (2001) Electrochim Acta 46:4258
Hammache H, Makhloufi L, Saidani B (2003) Corros Sci 45:2031
Nguyen Thi Le H, Garcia B, Deslouis C, Le Xuan Q (2001) Electrochim Acta 46:26
Nguyen Thi Le H, Garcia B, Deslouis C, Le Xuan Q (2002) J Electroanal Chem 32:105
Hien NTL, Garcia B, Pailleret A, Deslouis C (2005) Electrochim Acta 50:1747
Hammache H, Makhloufi L, Saidani B (2003) Corros Sci 45:2031
Garcia B, Lamzoudl A, Piller E, Nguyen Thi Le H, Deslous C (2002) J Electrochem Soc 149:B560
Lapkowski M, Biden G, Fournier M (1991) Synth Met 41:407
Dong S, Jin W (1993) J Eletroanal Chem 354:87
Cheng SA, Otero TF (2002) Synth Met 129:53
Ohtsuka T, Wakabayashi T, Einaga H (1994) J Electroanal Chem 377:107
Takahashi M, Tsuchida T, Ohtsuka T (1996) Thin Solid Films 280:124
Ohtsuka T, Wakabayashi T, Einaga H (1996) Synth Met 79:235
Acknowledgement
The present work was partially supported by ISIJ Research Promotion Grant of the Iron and Steel Institute of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohtsuka, T., Iida, M. & Ueda, M. Polypyrrole coating doped by molybdo-phosphate anions for corrosion prevention of carbon steels. J Solid State Electrochem 10, 714–720 (2006). https://doi.org/10.1007/s10008-006-0116-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-006-0116-0