Abstract
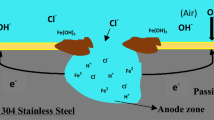
Pit morphology of Inconel alloy 600 in sulphate (SO4 2-), nitrate (NO3 -) and bicarbonate (HCO3 -) ion-containing 0.5 M sodium chloride (NaCl) solution was analysed in terms of fractal geometry as functions of solution temperature and anion concentration using the potentiostatic current transient technique, scanning electron microscopy, image analysis and ac-impedance spectroscopy. Potentiostatic current transients revealed that the pitting corrosion is facilitated by the increase in solution temperature, irrespective of anion additives, and that it is hindered by the increase in NO3 - and HCO3 - ion concentration, regardless of solution temperature. Above 60 °C, it was also found that the addition of SO4 2- ions impedes pit initiation, but enhances pit growth. The value of fractal dimension D f of the pits increased with increasing solution temperature and with decreasing NO3 - and HCO3 - ion concentration. Moreover, the value of D f increased above 60 °C with increasing SO4 2- ion concentration. This is caused by the increase in the ratio of pit perimeter to pit area, implying the formation of pits with micro-branched shape due to the acceleration of the local attack in the pits. From the decrease of the depression parameter with increasing solution temperature, it is inferred that the roughness of the pits increased with increasing solution temperature. In addition, the depression parameter was found to increase with increasing NO3 - and HCO3 - ion concentration. But, above 60 °C, in the case of SO4 2- ion addition, the depression parameter decreased with increasing SO4 2- ion concentration. From the experimental findings, the three-dimensional pit morphology is discussed in terms of the values of D f of the pits and the depression parameter, with respect to anion concentration and solution temperature.













Similar content being viewed by others
References
Ho JT, Yu GP (1992) Corrosion 48:147
Choi D, Was GS (1992) Corrosion 48:292
Chang MY, Yu GP (1993) J Nuc Mat 202:145
Bohni H, Uhlig HH (1969) J Electrochem Soc 116:906
Curley-Fiorino ME, Schmid GM (1980) Corros Sci 20:313
Reiser DB, Alkire RC (1984) Corros Sci 24:579
Alkire RC, Wong KP (1988) Corros Sci 28:411
Was GS, Choi D (1992) Corrosion 48:103
Mankowski G, Duthil JP, Giusti A (1997) Corros Sci 39:27
Park JJ, Pyun SI, Lee WJ, Kim HP (1999) Corrosion 55:380
Pyun SI, Na KH, Lee WJ, Park JJ (2000) Corrosion 56:1015
Pyun SI, Na KH, Park JJ (2000) J Solid State Electrochem 5:473
Park JJ, Pyun SI (2003) Corros Sci 45:995
Mandelbrot BB, Passoja DE, Paullay AJ (1984) Nature 308:721
Costa JM, Sagues F, Vilarasa M (1991) Corros Sci 32:665
Proost J, Baklanov M, Verbeeck R, Maex K (1998) J Solid State Electrochem 2:150
Shin HC, Go JY (2001) private communication, KAIST, Daejeon, Republic of Korea
Feder J (1988) Fractals. Plenum, New York, p 200
Bae JS, Pyun SI (1994) J Mat Sci Lett 13:573
Kolics A, Polkinghorne JC, Wieckowski A (1998) Electrochim Acta 43:2605
Pyun SI, Moon SM (1999) J Solid State Electrochem 3:331
Kobayashi Y, Virtanen S, Bohni H (2000) J Electrochem Soc 147: 155
Linke WF (1958) Solubilities, inorganic and metal organic compounds: a compilation of solubility data from the periodical literature. Van Nostrand, Princeton, NJ, p 1208, 1219
David DL (1985) Handbook of aqueous electrolyte solutions. Wiley, New York, p 240
Normant F, De Walle AV (1996) Cartographica 33:1
Macdonald JR (1987) Impedance spectroscopy. Wiley, New York, p 17
Piela B, Worna PK (1995) J Electroanal Chem 388:69
Pajkossy T, Nyikos (1986) J Electrochem Soc 133:2061
Halsey TC (1987) Phys Rev A 35:3512
Rammelt U, Reinhard G (1987) Corros Sci 27:373
Hill RM, Dissado LA (1988) Solid State Ionics 26:295
Mulder WH, Sluyters JH (1988) Electrochim Acta 33:303
Pajkossy T (1994) J Electroanal Chem 364:111
Acknowledgements
One of the authors (JJP) would like to thank to Mr. J.-S. Oh at the CIERL, KAIST for his great help with electrochemical experiments. This work was partly supported by the Brain Korea 21 project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pyun, SI., Park, JJ. Fractal analysis of pit morphology of Inconel alloy 600 in sulphate, nitrate and bicarbonate ion-containing sodium chloride solution at temperatures of 25–100 °C. J Solid State Electrochem 8, 296–307 (2004). https://doi.org/10.1007/s10008-003-0453-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-003-0453-1