Abstract
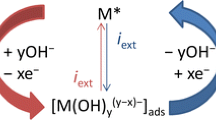
Gold is the noblest of metals and it is widely assumed that the surface of a gold electrode only begins to undergo oxidation in aqueous media at ca. 1.36 V (RHE) in acid or ca. 1.25 V in base. However, recent work in this laboratory has shown that, following thermal activation, gold atoms at the surface of a gold electrode may undergo oxidation in acid solution at potentials as low as 0.3 V. In the present work, in which the gold was allowed to cool rapidly from the molten state, oxidation responses were observed for gold in base at E<0.0 V. Such unusual behaviour is attributed to low gold-atom lattice stabilization energy plus the operation of a super-Nernstian E/pH effect. It appears, as outlined recently for silver, that a range of surface active states are available for metals; these give rise to a range of premonolayer oxidation and multilayer hydrous oxide reduction responses; such non-equilibrium surface states are often quite important with regard to electrocatalysis.







Similar content being viewed by others
References
Liebermann HH (1993) Rapidly solidified alloys. Dekker, New York
Reed-Hill RE, Abbaschian R (1992) Physical metallurgy principles, 3rd edn. PWS–Kent, Boston
Suryanarayana C (1991) Rapid solidification. In: Cahn R (ed) Materials science and technology, a comprehensive treatment, vol 15. VCH, Weinheim, pp 57–110
Cahn RW (1993) Background to rapid solidification processing. In: Liebermann HH (ed) Rapidly solidified alloys. Dekker, New York, p 1
Burke LD, Ahern AJ, O'Mullane AP (2002) Gold Bull 35:3
Taylor HS (1925) Proc R Soc Lond Ser A 108:105
von Oertzen A, Rotermund HH, Mikhailov AS, Ertl G (2000) J Phys Chem B 104:3155
Dieluweit S, Giesen M (2002) J Electroanal Chem 524–525:194
Buckley DN, Ahmed S (2003) Electrochem Solid-State Lett 6:C33–37
Pletcher D (1984) J Appl Electrochem 14:403
Bond GC, Thompson DT (1999) Catal Rev 41:319
Burke LD, Nugent PF (1997) Gold Bull 30:43
Burke LD, Nugent PF (1997) Gold Bull 31:39
Beltowska-Brzezinska M, Heitbaum J (1985) J Electroanal Chem 183:167
Beltowska-Brzezinska M, Luczak T, Holze R (1997) J Appl Electrochem 27:999
Ahern AJ, Nagle LC, Burke LD (2002) J Solid State Electrochem 6:451
Savinova ER, Kraft P, Pettinger B, Doblhofer K (1997) J Electroanal Chem 430:47
Strehblow H-H, Maurice V, Marcus P (2001) Electrochim Acta 46:3755
Desilvestro J, Weaver MJ (1986) J Electroanal Chem 209:373
Härtinger S, Pettinger B, Doblhofer K (1995) J Electroanal Chem 397:335
Zemlyanov DY, Savinova ER, Scheybal A, Doblhofer K, Schlögl R (1998) Surf Sci 418:441
Savinova ER, Zemlyanov D, Pettinger B, Scheybal A, Schlögl R, Doblhofer K (2000) Electrochim Acta 46:175
Kunze J, Maurice V, Klein LH, Strehblow H-H, Marcus P (2001) J Phys Chem B 105:4263
Shaikhutdinov ShK, Savinova ER, Scheybal A, Doblhofer K, Schögl R (2001) J Electroanal Chem 500:208
Burke LD, O'Mullane AP (2000) J Solid State Electrochem 4:285
Pourbaix M (1966) Atlas of electrochemical equilibria in aqueous media. Pergamon, Oxford
Burke LD, Lyons MEG (1986) Electrochemistry of hydrous oxide films. In: White RE, Bockris JO'M, Conway BE (eds) Electroanalytical chemistry, vol 18. Plenum, New York, pp 169–248
Considine DM (ed) (1995) Van Nostrand's scientific encyclopedia, 8th edn. Van Nostrand Reinhold, New York, p 36
Burke LD, Hurley LM, Lodge VE, Mooney MB (2001) J Solid State Electrochem 5:250
Burke LD, Hurley LM (2002) J Solid State Electrochem 6:101
Burke LD, O'Mullane AP, Lodge VE, Mooney MB (2001) J Solid State Electrochem 5:319
Srinivasan S, Gileadi E (1966) Electrochim Acta 11:321
Burke LD, Buckley DT (1996) J Electroanal Chem 405:101
Burke LD, Nugent PF (1998) J Electroanal Chem 444:19
Burke LD, Casey JK, Morrissey JA (1993) Electrochim Acta 38:897
Paliteiro C, Correia E (2000) J Electrochem Soc 147:3445
Paliteiro C (1994) Electrochim Acta 39:1633
Haruta M, Yamada N, Kobayashi T, Iijima S (1989) J Catal 115:301
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. Dr. Wolf Vielstich on the occasion of his 80th birthday in recognition of his numerous contributions to interfacial electrochemistry
Rights and permissions
About this article
Cite this article
Burke, L.D., Moran, J.M. & Nugent, P.F. Cyclic voltammetry responses of metastable gold electrodes in aqueous media. J Solid State Electrochem 7, 529–538 (2003). https://doi.org/10.1007/s10008-003-0359-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-003-0359-y