Abstract
Context
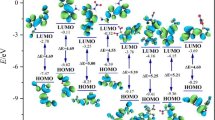
Various 7H,7′H-[6,6′-bi[1,2,4]triazolo[4,3-b][1,2,4]triazole]-3,3′,7,7′-tetramine (A) based nitrogen-rich energetic salts were designed and their properties explored. All energetic salts possess relatively high nitrogen content (> 48%), positive heats of formation (> 429 kJ/mol) and stability owing to a significant contribution from fused backbone. The cationic component shows a very high heat of formation (2516 kJ/mol); therefore, it is highly suitable for enthalpy enhancement in new energetic salts. The cation was paired with the energetic anions nitrate (NO3−), perchlorate (ClO4−), dinitromethanide (CH(NO2)2−), trinitromethanide (C(NO2)3−), nitroamide (NHNO2−), and dinitroamide (N(NO2)2−) to improve oxygen balance and detonation performance. Designed salts show moderate detonation velocities (7.9–8.7 km/s) and pressures (23.8 − 33.1 GPa). The distribution of frontier molecular orbitals, molecular electrostatic surface potentials, QTAIM topological properties, and noncovalent interactions of designed salts were simulated to understand the electronic structures, charge distribution in molecules, hydrogen bonding, and other nonbond interactions. The predicted safety factor (SF) and impact sensitivity (H50) of designed salts suggest their insensitivity to mechanical stimuli. This work explored the 7H,7′H-[6,6′-bi[1,2,4]triazolo[4,3-b][1,2,4]triazole]-3,3′,7,7′-tetramine as a suitable cationic component which could be promising and serve exemplarily in energetic materials.
Methods
The optimization and energy calculations of all the designed compounds were carried out at the B3LYP/6–311 + + G(d,p) and M06-2X/def2-TZVPP levels, utilizing the Gaussian software package. The molecular surface electrostatic potential, quantum theory of atoms in molecules (QTAIM), reduced density gradient (RDG), and noncovalent interaction (NCI) analysis were performed by employing Multiwfn software. The EXPLO5 (v 7.01) thermochemical code and PILEM web application were used to predict the detonation properties.
Graphical abstract









Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Pagoria PF, Lee GS, Mitchell AR, Schmidt RD (2002) A review of energetic materials synthesis. Thermochim Acta 384:187–204
Zhang J, Zhou J, Bi F, Wang B (2020) Energetic materials based on poly furazan and furoxan structures. Chin Chem Lett 31:2375–2394
Gao H, Zhang Q, Shreeve JM (2020) Fused heterocycle-based energetic materials (2012–2019). J Mater Chem A 8:4193–4216
Wu JT, Xu J, Li W, Li HB (2020) Coplanar fused heterocycle-based energetic materials. Propellants Explos Pyrotech 45:536
Manzoor S, Tariq Q, Yin X, Zhang JG (2021) Nitro-tetrazole based high performing explosives: recent overview of synthesis and energetic properties. Def Technol 17:1995–2010
He P, Zhang JG, Yin X, Wu JT, Wu L, Zhou ZN, Zhang TL (2016) Energetic salts based on tetrazole N-oxide. Chem Eur J 22:7670–7685
Tang J, Yang H, Cui Y, Cheng G (2021) Nitrogen-rich tricyclic-based energetic materials. Mater Chem Front 5:7108–7118
Banik S, Yadav AK, Kumar P, Ghule VD, Dharavath S (2022) Unfolding the chemistry of FOX-7: unique energetic material and precursor with numerous possibilities. Chem Eng J 431:133378
Zhang S, Gao Z, Lan D, Jia Q, Liu N, Zhang J, Kou K (2020) Recent advances in synthesis and properties of nitrated-pyrazoles based energetic compounds. Molecules 25:3475
Gao H, Shreeve JM (2011) Azole-based energetic salts. Chem Rev 111:7377–7436
Klapötke TM (2012) Chemistry of high-energy materials. Boston, De Gruyter, Berlin, p 2012
Keshavarz MH, Klapötke TM (2021) The properties of energetic materials: sensitivity, physical and thermodynamic properties. Boston, De Gruyter, Berlin
Wu Q, Hu Q, Tan L, Zhu W (2023) New cage super insensitive high energy materials constructed by the Diels-Alder reaction based on nitroazoles: a DFT study. Mater Chem Phys 298:127461
Wang Y, Hu L, Pang S, Shreeve JM (2023) Nitroimino as an energetic group in designing energetic materials for practical use, a tautomerism from nitroamino. J Mater Chem A 11:13876–13888
Wu Q, Teng Z, Zhu W (2022) Desensitizing high energy materials HMX and CL-20 by the smallest all carbon compound cyclo[18]carbon: a DFT study. J Mater Sci 57:10197–10212
Wu Q, Sun T, Tan L, Zhu W (2022) First principle study and Hirshfeld surface analysis on the effect of type, number, and position of small molecules on the structural stability and optical property of a powerful energetic crystal 6-nitro-7-azido-pyrazol[3,4-d][1,2,3]triazine-2-oxide. Mater Adv 3:1035–1046
Yang J, Bai T, Guan J, Li M, Zhen Z, Dong X, Wang Y, Wang Y (2023) Novel fluorine-containing energetic materials: how potential are they? A computational study of detonation performance. J Mol Model 29:228
Xiao T, Chen J, Xu J, Ma P, Ma C (2023) Theoretical insight into different energetic groups on the performance of energetic materials 2,5,7,9-tetranitro-2,5,7,9-tetraazabicyclo[4,3,0]nonane-8-one. J Mol Model 29:231
Wu Q, Yan G, Tan L, Zhu W, Zhou Y (2023) Theoretical design of new insensitive high energy metal complexes based on the double fused-ring insensitive ligands strategy. J Mol Model 29:84
Tang Y, He C, Imler GH, Parrish DA, Shreeve JM (2017) High-performing and thermally stable energetic 3,7-diamino-7H-[1,2,4]triazolo[4,3-b][1,2,4]triazole derivatives. J Mater Chem A 5:6100–6105
Nie X, Lei C, Xiong H, Cheng G, Yang H (2020) Methylation of a triazole-fused framework to create novel insensitive energetic materials. Energ Mater Front 1:165–171
Lei C, Yang H, Zhang Q, Cheng G (2021) Synthesis of nitrogen-rich and thermostable energetic materials based on hetarenecarboxylic acids. Dalton Trans 50:14462–14468
Gaussian 09, Revision E.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian Inc, Wallingford, CT
Lu T (2012) Chen F Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Ghule VD (2012) Computational studies on energetic properties of trinitro-substituted imidazole–triazole and pyrazole–triazole derivatives. J Phys Chem A 116:9391–9397
Ghule VD (2013) Computational screening of nitrogen-rich energetic salts based on substituted triazine. J Phys Chem C 117:16840–16849
Nirwan A, Ghule VD (2018) Estimation of heats of formation for nitrogen-rich cations using G3, G4, and G4 (MP2) theoretical methods. Theoret Chem Acc 137:115
Maan A, Ghule VD, Dharavath S (2023) Computational manifestation of nitro-substituted Tris(triazole): understanding the impact of isomerism on performance-stability parameters. J Phys Chem A 127:6467–6475
Devi R, Maan A, Ghule VD, Dharavath S (2023) Functionalization of fused imidazole-oxadiazole, triazole-oxadiazole and tetrazole-oxadiazole skeletons: search for stable and potential energetic materials. Comput Theor Chem 1229:114352
Sharma K, Maan A, Ghule VD, Dharavath S (2023) Azo-bridged triazole macrocycles: computational design, energy content, performance, and stability assessment. J Phys Chem A 127:10128–10138
Bondarchuk SV (2021) Diazoamination: a simple way to enhance detonation performance of aminoaromatic and aminoheterocyclic energetic materials. FirePhysChem 1:97–102
Curtiss LA, Redfern PC, Raghavachari K (2007) Gaussian-4 theory. J Chem Phys 126:084108
Curtiss LA, Redfern PC, Raghavachari K (2007) Gaussian-4 theory using reduced order perturbation theory. J Chem Phys 127:124105
Dorofeeva OV, Ryzhova ON, Suntsova MA (2013) Accurate prediction of enthalpies of formation of organic azides by combining G4 theory calculations with an isodesmic reaction scheme. J Phys Chem A 117:6835–6845
Dorofeeva OV, Suntsova MA (2015) Enthalpy of formation of CL-20. Comput Theor Chem 1057:54–59
Jenkins HDB, Tudela D, Glasser L (2002) Lattice potential energy estimation for complex ionic salts from density measurements. Inorg Chem 41:2364–2367
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbé A (2009) An electrostatic interaction correction for improved crystal density prediction. Mol Phys 107:2095–2101
Politzer P, Martinez J, Murray JS, Concha MC (2010) An electrostatic correction for improved crystal density predictions of energetic ionic compounds. Mol Phys 108:1391–1396
Muravyev NV, Wozniak DR, Piercey DG (2022) Progress and performance of energetic materials: open dataset, tool, and implications for synthesis. J Mater Chem A 10:11054–11073
Zhang J, Lu T (2021) Efficient evaluation of electrostatic potential with computerized optimized code. Phys Chem Chem Phys 23:20323–20328
Wick CR, Clark T (2018) On bond-critical points in QTAIM and weak interactions. J Mol Model 24:1–9
Shahbazian S (2018) Why bond critical points are not “bond” critical points. Chem Eur J 24:5401–5405
Zhang X, Gong X (2014) Screening nitrogen-rich bases and oxygen-rich acids by theoretical calculations for forming highly stable salts. ChemPhysChem 15:2281–2287
Rozas I, Alkorta I, Elguero J (2000) Behavior of ylides containing N, O, and C atoms as hydrogen bond acceptors. J Am Chem Soc 122:11154–11161
Venkataramanan NS, Suvitha A, Kawazoe Y (2017) Intermolecular interaction in nucleobases and dimethyl sulfoxide/water molecules: a DFT, NBO, AIM and NCI analysis. J Mol Graph 78:48–60
Otero-de-la-Roza A, Johnson ER, García JC (2012) Revealing non-covalent interactions in solids: NCI plots revisited. Phys Chem Chem Phys 14:12165–12172
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506
Aihara J (1999) Weighted HOMO-LUMO energy separation as an index of kinetic stability for fullerenes. Theor Chem Acc 102:134–138
Zhang H, Cheung F, Zhao F, Cheng XL (2009) Band gaps and the possible effect on impact sensitivity for some nitro aromatic explosive materials. Int J Quant Chem 109:1547–1552
Qi-L Y, Zeman S (2012) Theoretical evaluation of sensitivity and thermal stability for high explosives based on quantum chemistry methods: a brief review. Int J Quantum Chem 113:1049–1061
Zhu W, Xiao H (2010) First-principles band gap criterion for impact sensitivity of energetic crystals: a review. Struct Chem 21:657–665
Zeman S, Jungová M (2016) Sensitivity and performance of energetic materials. Propellants Explos Pyrotech 41:426–451
Bondarchuk SV (2022) Chapter 9 - Interplay between chemical and mechanical factors. In Theor Comput Chem 22:195–213 (Molecular Modeling of the Sensitivities of Energetic Materials, edited by Mathieu D, Elsevier)
Muravyev NV, Meerov DB, Monogarov KA, Melnikov IN, Kosareva EK, Fershtat LL, Sheremetev AB, Dalinger IL, Fomenkov IV, Pivkina AN (2021) Sensitivity of energetic materials: evidence of thermodynamic factor on a large array of CHNOFCl compounds. Chem Eng J 421:129804
Keshavarz MH, Zali A, Shokrolahi A (2009) A simple approach for predicting impact sensitivity of polynitroheteroarenes. J Hazard Mater 166:1115–1119
Acknowledgements
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. Rimpi thank UGC-CSIR, Human Resource Development Group, Government of India for the Research Fellowship. Kalpana thanks the Council of Scientific &; Industrial Research (CSIR), India, for providing the Junior Research Fellowship (CSIR-HRDG Ref. No.: Sept/06/22(i)EU-V).
Author information
Authors and Affiliations
Contributions
Rimpi Devi: conceptualization, methodology, programming, validation, writing—original draft. Kalpana Sharma: conceptualization, investigation, writing—original draft. Vikas D. Ghule: investigation, methodology, funding acquisition, supervision, project administration, software, writing—original draft, writing—review and editing. Srinivas Dharavath: funding acquisition, software, writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Devi, R., Sharma, K., Ghule, V.D. et al. Bistriazolotriazole-tetramine: commendable energetic moiety and cation. J Mol Model 30, 98 (2024). https://doi.org/10.1007/s00894-024-05892-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-024-05892-6