Abstract
Context
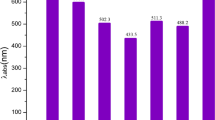
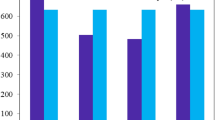
Various innovative molecules have been designed and explored for use in organic photovoltaics. In this study, we devised novel molecules (KZ1–KZ7) specifically for organic solar cells (OSCs). The newly formulated acceptor compounds possess a lower bandgap (Eg = 1.85–2.02), along with bathochromic shift (λmax = 713–788 nm) compared to the reference (Eg = 2.04 eV and λmax = 774 nm). Moreover, the FMO results identified the distinct charge transfer from HOMO to LUMO, which was strongly corroborated by the TDM maps. Similarly, the new designed molecules show less excitation energy (Ex = 1.31–1.54(gas)) than reference (Ex = 1.72). Likewise, all designed molecules (KZ1–KZ7) have demonstrated an analogous open circuit voltage (Voc) with the donor polymer PTB7-Th. All seven designed molecules (KZ1–KZ7) exhibited more fill factor ranging from 97.08 to 97.29 than reference 95.25 and PCE of between 8 and 20% at short circuit current densities of 9, 12, and 15 mA cm−2. Overall, the findings support that designed molecules can be potential molecules for future practical applications.
Methods
Geometric calculations were conducted with Gaussian 09W software, and the findings were visualized using Gauss View software. DFT and TD-DFT were employed to evaluate various parameters for R and designed molecules (KZ1–KZ7). Firstly, four functionals including B3LYP, CAM-B3LYP, MPW1PW91, and ωB97XD with 6-31G(d,p) DFT level were applied to R to decide the best level for results. After appropriate analysis, the MPW1PW91/6-31G(d,p) was selected for further examination by comparing the experimental and DFT-based absorption graphs of R. External and internal reorganization energy are the two main factors contributing to reorganization energy. External energy refers to changes in external environment, while internal energy deals with information related to internal geometrical symmetry or the internal environment. The effect of outside factors or external reorganizational energy is omitted because it creates too little change.








Similar content being viewed by others
Data availability
Data supporting the previously stated findings are available upon request. Queries should be directed to the relevant author.
References
Tajbakhsh M et al (2017) Molecular design of novel fullerene-based acceptors for enhancing the open circuit voltage in polymer solar cells. Journal of Physics and Chemistry of Solids 111:410–418
Adamo C, Barone V (1998) Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: the m PW and m PW1PW models. The Journal of chemical physics 108(2):664–675
Cui Y et al (2020) Single-junction organic photovoltaic cells with approaching 18% efficiency. Advanced Materials 32(19):1908205
Emmott CJ et al (2015) Organic photovoltaic greenhouses: a unique application for semi-transparent PV. Energy & environmental science 8(4):1317–1328
Fan B et al (2019) Achieving over 16% efficiency for single-junction organic solar cells. Science China Chemistry 62:746–752
Gasparini N et al (2018) The physics of small molecule acceptors for efficient and stable bulk heterojunction solar cells. Advanced Energy Materials 8(12):1703298
Kebiroglu H et al Investigation of propyphenazone molecule by quantum chemical methods. Journal of Physical Chemistry 5(2):40–48
Köse M (2012) Evaluation of acceptor strength in thiophene coupled donor–acceptor chromophores for optimal design of organic photovoltaic materials. The Journal of Physical Chemistry A 116(51):12503–12509
Li C et al (2019) Asymmetric nonfullerene small molecule acceptors for organic solar cells. Advanced Energy Materials 9(25):1900999
Lai H et al (2019) Isomer-free: precise positioning of chlorine-induced interpenetrating charge transfer for elevated solar conversion. iScience 17:302–314
Liang N et al (2017) New developments in non-fullerene small molecule acceptors for polymer solar cells. Materials Chemistry Frontiers 1(7):1291–1303
Liao X et al (2022) The synergistic effects of central core size and end group engineering on performance of narrow bandgap nonfullerene acceptors. Chemical Engineering Journal 435:135020
Luo Z et al (2020) Altering the positions of chlorine and bromine substitution on the end group enables high-performance acceptor and efficient organic solar cells. Advanced Energy Materials 10(44):2002649
Meng L et al (2018) Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 361(6407):1094–1098
Qin J et al (2020) Over 16% efficiency from thick-film organic solar cells. RRL Solar 65(23):1979–1982
Ravishankar E et al (2020) Achieving net zero energy greenhouses by integrating semitransparent organic solar cells. Joule 4(2):490–506
Reichenbächer K, Süss HI, Hulliger JJCSR (2005) Fluorine in crystal engineering—“the little atom that could”. Chemical Society Reviews 34(1):22–30
Shi X et al (2018) Terthieno [3, 2-b] thiophene (6T) based low bandgap fused-ring electron acceptor for highly efficient solar cells with a high short-circuit current density and low open-circuit voltage loss. Advanced Energy Materials 8(12):1702831
Sun H et al (2021) Regioregular narrow-bandgap n-type polymers with high electron mobility enabling highly efficient all-polymer solar cells. Advanced Materials 33(37):2102635
Ganji MD et al (2016) Tuning the LUMO level of organic photovoltaic solar cells by conjugately fusing graphene flake: a DFT-B3LYP study. Physica E: Low-dimensional Systems and Nanostructures 81:108–115
Mehboob MY et al (2022) Efficient designing of half-moon-shaped chalcogen heterocycles as non-fullerene acceptors for organic solar cells. Journal of Molecular Modeling 28(5):125
Mehboob MY et al (2021) Designing of benzodithiophene core-based small molecular acceptors for efficient non-fullerene organic solar cells. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 244:118873
Ganji MD, Rezvani M, Tanreh S (2021) Characterization and theoretical modeling of solar cells. Fundamentals of Solar Cell Design:169–215
Yao H et al (2017) Design, synthesis, and photovoltaic characterization of a small molecular acceptor with an ultra-narrow band gap. Angewandte Chemie International Edition 56(11):3045–3049
Xiao Z et al (2017) 26 mA cm−2 Jsc from organic solar cells with a low-bandgap nonfullerene acceptor. Science Bulletin 62(22):1494–1496
Liao X et al (2022) The synergistic effects of central core size and end group engineering on performance of narrow bandgap nonfullerene acceptors. Chemical Engineering Journal 435:135020
Bachoc F, Helbert C, Picheny V (2020) Gaussian process optimization with failures: classification and convergence proof. Journal of Global Optimization 78:483–506
Dennington, R., T. Keith, and J.J.I. Millam, Wallingford, GaussView 5.0, Gaussian. 2008. 20.
Civalleri B et al (2008) B3LYP augmented with an empirical dispersion term (B3LYP-D*) as applied to molecular crystals. CrystEngComm 10(4):405–410
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chemical physics letters 393(1-3):51–57
Adamo C, Barone V (1998) Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: the m PW and m PW1PW models. The Journal of chemical physics 108(2):664–675
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Physical Chemistry Chemical Physics 10(44):6615–6620
Bachoc F, Helbert C, Picheny V (2020) Gaussian process optimization with failures: classification and convergence proof. Journal of Global Optimization 78(3):483–506
Hussain R et al (2020) Enhancement in photovoltaic properties of N,N-diethylaniline based donor materials by bridging core modifications for efficient solar cells. ChemistrySelect 5(17):5022–5034
Afzal Z et al (2020) Designing indenothiophene-based acceptor materials with efficient photovoltaic parameters for fullerene-free organic solar cells. Journal of molecular modeling 26(6)
Rezvani M et al (2018) DFT/TD-semiempirical study on the structural and electronic properties and absorption spectra of supramolecular fullerene-porphyrine-metalloporphyrine triads based dye-sensitized solar cells. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 194:57–66
Ayub AR et al (2023) Designing of asymmetric non-fullerene based acceptor materials by re-modification of π-spacers with PCE > 20% for organic solar cell. Optik 278:170602
Moghim MT et al (2022) Computational investigation on the geometry and electronic structures and absorption spectra of metal-porphyrin-oligo-phenyleneethynylenes-[60] fullerene triads. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 280:121488
Janjua MRSA et al (2022) Computational engineering to enhance the photovoltaic by end-capped and bridging core alterations: empowering the future with solar energy through synergistic effect in D-A materials. International Journal of Quantum Chemistry 122(1):e26821
Hassan T et al (2022) Development of non-fused acceptor materials with 3D-interpenetrated structure for stable and efficient organic solar cells. Materials Science in Semiconductor Processing 151:107010
Ans M et al (2019) Designing indacenodithiophene based non-fullerene acceptors with a donor–acceptor combined bridge for organic solar cells. RSC advances 9(7):3605–3617
Rasool A et al (2021) Bithieno thiophene-based small molecules for application as donor materials for organic solar cells and hole transport materials for perovskite solar cells. ACS Omega 7(1):844–862
Iqbal MMA et al (2023) Designing efficient AD-A1-DA type fullerene free acceptor molecules with enhanced power conversion efficiency for solar cell applications. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy 285:121844
Iqbal MMA et al (2022) Quinoxaline based unfused non-fullerene acceptor molecules with PTB7-Th donor polymer for high performance organic solar cell applications. Journal of Molecular Graphics and Modelling 114:108181
Ans M et al (2018) Designing three-dimensional (3D) non-fullerene small molecule acceptors with efficient photovoltaic parameters. ChemistrySelect 3(45):12797–12804
Ans M et al (2019) Designing indacenodithiophene based non-fullerene acceptors with a donor–acceptor combined bridge for organic solar cells. RSC advances 9(7):3605–3617
Iqbal MMA et al (2022) High electron mobility due to extra π-conjugation in the end-capped units of non-fullerene acceptor molecules: a DFT/TD-DFT-based prediction. Journal of Molecular Modeling 28(9):278
Iqbal MMA, Mehboob MY, Hassan T (2022) Theoretical study of the structure-activity relationship of the S-shaped acceptor molecules for organic solar cell applications. Materials Science in Semiconductor 148:106763
Iqbal MMA et al (2021) Synergistic effects of fluorine, chlorine and bromine-substituted end-capped acceptor materials for highly efficient organic solar cells. Computational and Theoretical Chemistry 1202:113335
Zafar F et al (2021) End-capped engineering of truxene core based acceptor materials for high performance organic solar cells: theoretical understanding and prediction. Optical and Quantum Electronics 53:1–24
Ghahramanpour M, Jamehbozorgi S, Rezvani M (2020) The effect of encapsulation of lithium atom on supramolecular triad complexes performance in solar cell by using theoretical approach. Adsorption 26:471–489
Kanwal N et al (2022) DFT based modeling of asymmetric non-fullerene acceptors for high-performance organic solar cell. Optical and Quantum Electronics 54(9):546
Rehman, F.U et al (2023)., Approach toward low energy loss in symmetrical nonfullerene acceptor molecules inspired by insertion of different π-spacers for developing efficient organic solar cells. ACS Omega 8, 46, 43792–43812
Hussain R et al (2023) Designing of silolothiophene-linked triphenylamine-based hole transporting materials for perovskites and donors for organic solar cells-a DFT study. Solar Energy 253:187–198
Rehman, F.U (2023) et al., High-efficiency and low-energy-loss organic solar cells enabled by tuning the end group modification of the terthiophene-based acceptor molecules to enhance photovoltaic properties. ACS Omega 42492–42510 2023.
Ans M et al (2019) Opto-electronic properties of non-fullerene fused-undecacyclic electron acceptors for organic solar cells. Computational Materials Science 159:150–159
Ayub AR et al (2023) Designing of asymmetric non-fullerene based acceptor materials by re-modification of π-spacers with PCE > 20% for organic solar cell. Optik 278:170602
Acknowledgements
The authors are highly acknowledged to the University of Okara, for providing technical support for this project. We are also thankful to the COMSAT University Islamabad, Abbottabad, Pakistan, for providing computational facility support.
Author information
Authors and Affiliations
Contributions
KA: validation, visualization, formal analysis, writing. TH: visualization, validation, methodology, writing—original draft. MMAI: conceptualization, project administration, data curation, resources, supervision, software, paper editing. RH: data analysis, data curation, visualization.
Corresponding authors
Ethics declarations
Conflict of interest
All authors have declared no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Atiq, K., Iqbal, M.M.A., Hassan, T. et al. An efficient end-capped engineering of pyrrole-based acceptor molecules for high-performance organic solar cells. J Mol Model 30, 13 (2024). https://doi.org/10.1007/s00894-023-05799-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05799-8