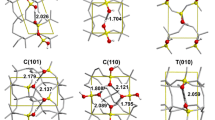
Abstract
Surface hydroxylation has been extensively studied over the years for a variety of applications, and studies involving hydroxylation of different silica surfaces are still carried out due to the interesting properties obtained from those modified surfaces. Although a number of theoretical studies have been employed to evaluate details on the hydroxylation phenomenon on silica (SiO2) surfaces, most of these studies are based on computationally expensive models commonly based on extended systems. In order to circumvent such an aspect, here we present a low-cost theoretical study on the SiO2 hydroxylation process aiming to evaluate aspects associated with water-SiO2 interaction. Details about local reactivity, chemical softness, and electrostatic potential were evaluated for SiO2 model substrates in the framework of the density functional theory (DFT) using a molecular approach. The obtained results from this new and promising approach were validated and complemented by fully atomistic reactive molecular dynamics (FARMD) simulations. Furthermore, the implemented approach proves to be a powerful tool that is not restricted to the study of hydroxylation, opening a promising route for low computational cost to analyze passivation and anchoring processes on a variety of oxide surfaces.
Graphical abstract








Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Pujari SP, Scheres L, Marcelis ATM, Zuilhof H (2014) Covalent surface modification of oxide surfaces. Angew Chem Int Ed 53:6322–6356. https://doi.org/10.1002/anie.201306709
Armistead CG, Tyler AJ, Hambleton FH et al (1969) Surface hydroxylation of silica. J Phys Chem 73:3947–3953. https://doi.org/10.1021/j100845a065
Hanawa T (2011) A comprehensive review of techniques for biofunctionalization of titanium. J Periodontal Implant Sci 41:263–272. https://doi.org/10.5051/jpis.2011.41.6.263
Tamura H, Mita K, Tanaka A, Ito M (2001) Mechanism of hydroxylation of metal oxide surfaces. J Colloid Interface Sci 243:202–207. https://doi.org/10.1006/jcis.2001.7864
Asakuma N, Fukui T, Toki M et al (2003) Photoinduced hydroxylation at ZnO surface. Thin Solid Films 445:284–287. https://doi.org/10.1016/S0040-6090(03)01162-3
Hashimoto K, Irie H, Fujishima A (2005) TiO2 photocatalysis: a historical overview and future prospects. Jpn J Appl Phys 44:8269. https://doi.org/10.1143/JJAP.44.8269
Lohbauer U, Zipperle M, Rischka K et al (2008) Hydroxylation of dental zirconia surfaces: characterization and bonding potential. J Biomed Mater Res B Appl Biomater 87B:461–467. https://doi.org/10.1002/jbm.b.31126
Sneh O, George SM (1995) Thermal stability of hydroxyl groups on a well-defined silica Surface. J Phys Chem 99:4639–4647. https://doi.org/10.1021/j100013a039
Ide MS, Davis RJ (2014) The important role of hydroxyl on oxidation catalysis by gold nanoparticles. Acc Chem Res 47:825–833. https://doi.org/10.1021/ar4001907
Robert D, Malato S (2002) Solar photocatalysis: a clean process for water detoxification. Sci Total Environ 291:85–97. https://doi.org/10.1016/s0048-9697(01)01094-4
Gentleman MM, Ruud JA (2010) Role of hydroxyls in oxide wettability. Langmuir 26:1408–1411. https://doi.org/10.1021/la903029c
Gomes OP, Azevedo Neto NF, Bronze-Uhle ES et al (2019) 3-Mercaptopropionic acid functionalization of titanium dioxide thin films. Mater Chem Phys 223:32–38. https://doi.org/10.1016/j.matchemphys.2018.10.041
Gomes OP, Feltran GS, Ferreira MR et al (2020) A novel BSA immobilizing manner on modified titanium surface ameliorates osteoblast performance. Colloids Surf B Biointerfaces 190:110888. https://doi.org/10.1016/j.colsurfb.2020.110888
Du J, Cormack AN (2005) Molecular dynamics simulation of the structure and hydroxylation of silica glass surfaces. J Am Ceram Soc 88:2532–2539. https://doi.org/10.1111/j.1551-2916.2005.00352.x
Rimsza JM, Du J (2015) Ab initio molecular dynamics simulations of the hydroxylation of nanoporous silica. J Am Ceram Soc 98:3748–3757. https://doi.org/10.1111/jace.13731
Guan K (2005) Relationship between photocatalytic activity, hydrophilicity and self-cleaning effect of TiO2/SiO2 films. Surf Coat Technol 191:155–160. https://doi.org/10.1016/j.surfcoat.2004.02.022
Bunker BC (1994) Molecular mechanisms for corrosion of silica and silicate glasses. J Non-Cryst Solids 179:300–308. https://doi.org/10.1016/0022-3093(94)90708-0
Fogarty JC, Aktulga HM, Grama AY et al (2010) A reactive molecular dynamics simulation of the silica-water interface. J Chem Phys 132:174704. https://doi.org/10.1063/1.3407433
Iler RK (1979) The chemistry of silica: solubility, polymerization, colloid and surface properties, and biochemistry. Wiley, New York
Kurkjian CR, Krause JT, Paek UC (1982) Tensile strength characteristics of “perfect” silica fibers. J Phys Colloques 43:585–586. https://doi.org/10.1051/jphyscol:19829115
Gaigeot M-P, Sprik M, Sulpizi M (2012) Oxide/water interfaces: how the surface chemistry modifies interfacial water properties. J Phys Condens Matter 24:124106. https://doi.org/10.1088/0953-8984/24/12/124106
Sulpizi M, Gaigeot M-P, Sprik M (2012) The silica–water interface: how the silanols determine the surface acidity and modulate the water properties. J Chem Theory Comput 8:1037–1047. https://doi.org/10.1021/ct2007154
He Y, Cao C, Trickey SB, Cheng H-P (2008) Predictive first-principles simulations of strain-induced phenomena at water-silica nanotube interfaces. J Chem Phys 129:011101. https://doi.org/10.1063/1.2953457
Michalske TA, Freiman SW (1982) A molecular interpretation of stress corrosion in silica. Nature 295:511–512. https://doi.org/10.1038/295511a0
Gierada M, Petit I, Handzlik J, Tielens F (2016) Hydration in silica based mesoporous materials: a DFT model. Phys Chem Chem Phys 18:32962–32972. https://doi.org/10.1039/C6CP05460A
Hair ML, Hertl W (1969) Adsorption on hydroxylated silica surfaces. J Phys Chem 73:4269–4276. https://doi.org/10.1021/j100846a039
Tielens F, Gierada M, Handzlik J, Calatayud M (2020) Characterization of amorphous silica based catalysts using DFT computational methods. Catal Today 354:3–18. https://doi.org/10.1016/j.cattod.2019.03.062
Yeon J, van Duin ACT (2016) ReaxFF molecular dynamics simulations of hydroxylation kinetics for amorphous and nano-silica structure, and its relations with atomic strain energy. J Phys Chem C 120:305–317. https://doi.org/10.1021/acs.jpcc.5b09784
Zhdanov SP, Kosheleva LS, Titova TI (1987) IR study of hydroxylated silica. Langmuir 3:960–967. https://doi.org/10.1021/la00078a014
Mankad V, Jha PK (2016) First-principles study of water adsorption on α-SiO2 [110] surface. AIP Adv 6:085001. https://doi.org/10.1063/1.4960455
Nayak SK, Rao BK, Khanna SN, Jena P (1998) Atomic and electronic structure of neutral and charged SinOm clusters. J Chem Phys 109:1245–1250. https://doi.org/10.1063/1.476675
Allouche A-R (2011) Gabedit—A graphical user interface for computational chemistry softwares. J Comput Chem 32:174–182. https://doi.org/10.1002/jcc.21600
Schaftenaar G, Noordik JH (2000) Molden: a pre- and post-processing program for molecular and electronic structures*. J Comput Aided Mol Des 14:123–134. https://doi.org/10.1023/A:1008193805436
Keith TA, Millam JM (2016) GaussView. Semichen Inc., Shawnee Mission, KS
Stewart JJP (2016) MOPAC2016. Stewart computational chemistry, Colorado Springs, CO, USA. http://openmopac.net/
Frisch MJ, Trucks GW, Schlegel HB et al (2016) Gaussian 16. Gaussian Inc, Wallingford CT
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627. https://doi.org/10.1021/j100096a001
Vosko SH, Wilk L, Nusair M (2011) Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can J Phys. https://doi.org/10.1139/p80-159
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3094. https://doi.org/10.1021/cr9904009
Gałyńska M, Persson P (2014) Chapter Eight - Material Dependence of Water Interactions with Metal Oxide Nanoparticles: TiO2, SiO2, GeO2, and SnO2. In: Sabin JR (ed) Advances in Quantum Chemistry. Academic Press, pp 303–332. https://doi.org/10.1016/B978-0-12-800345-9.00008-8
Gomes OP, Batagin-Neto A, Lisboa-Filho PN (2021) The evaluation of anchoring processes and chemical stability of zwitterionic molecules via local reactivity indexes. Comput Mater Sci 193:110418. https://doi.org/10.1016/j.commatsci.2021.110418
Madhan Kumar A, Latthe SS, Sudhagar P et al (2015) In-situ synthesis of hydrophobic SiO2-PMMA composite for surface protective coatings: experimental and quantum chemical analysis. Polymer 77:79–86. https://doi.org/10.1016/j.polymer.2015.09.030
Martínez JJ, Silva L, Rojas HA et al (2017) Reductive amination of levulinic acid to different pyrrolidones on Ir/SiO2-SO3H: elucidation of reaction mechanism. Catal Today 296:118–126. https://doi.org/10.1016/j.cattod.2017.08.038
Yang W, Parr RG (1985) Hardness, softness, and the fukui function in the electronic theory of metals and catalysis. Proc Natl Acad Sci 82:6723–6726. https://doi.org/10.1073/pnas.82.20.6723
Weitao Y, Mortier WJ (1986) The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines. J Am Chem Soc 108:5708–5711. https://doi.org/10.1021/ja00279a008
Lascane LG, Oliveira EF, Galvão DS, Batagin-Neto A (2020) Polyfuran-based chemical sensors: identification of promising derivatives via DFT calculations and fully atomistic reactive molecular dynamics. Eur Polym J 141:110085. https://doi.org/10.1016/j.eurpolymj.2020.110085
Maia RA, Ventorim G, Batagin-Neto A (2019) Reactivity of lignin subunits: the influence of dehydrogenation and formation of dimeric structures. J Mol Model 25:228. https://doi.org/10.1007/s00894-019-4130-4
Plácido A, Bueno J, Barbosa EA et al (2020) The antioxidant peptide salamandrin-I: First bioactive peptide identified from skin secretion of Salamandra Genus (Salamandra salamandra). Biomolecules 10:512. https://doi.org/10.3390/biom10040512
Bulat FA, Murray JS, Politzer P (2021) Identifying the most energetic electrons in a molecule: the highest occupied molecular orbital and the average local ionization energy. Comput Theor Chem 1199:113192. https://doi.org/10.1016/j.comptc.2021.113192
Pearson RG (1963) Hard and soft acids and bases. J Am Chem Soc 85:3533–3539. https://doi.org/10.1021/ja00905a001
Melin J, Aparicio F, Subramanian V et al (2004) Is the fukui function a right descriptor of hard−hard interactions? J Phys Chem A 108:2487–2491. https://doi.org/10.1021/jp037674r
Chirlian LE, Francl MM (1987) Atomic charges derived from electrostatic potentials: a detailed study. J Comput Chem 8:894–905. https://doi.org/10.1002/jcc.540080616
Lloyd A, Cornil D, van Duin ACT et al (2016) Development of a ReaxFF potential for Ag/Zn/O and application to Ag deposition on ZnO. Surf Sci 645:67–73. https://doi.org/10.1016/j.susc.2015.11.009
Plimpton S (1995) Fast parallel algorithms for short-range molecular dynamics. J Comput Phys 117:1–19. https://doi.org/10.1006/jcph.1995.1039
Funding
This work as supported by the Brazilian National Council for Scientific and Technological Development (CNPq) (grant numbers 448310/2014–7 and 420449/2018–3), the São Paulo Research Foundation (FAPESP) (grant number 2019/09431–0), and the CEPID (grant number 2013/07296–2). This research was also supported by resources supplied by the Center for Scientific Computing (NCC/Grid-UNESP) of São Paulo State University (UNESP).
Author information
Authors and Affiliations
Contributions
The conception and design of the study were made by Orisson P. Gomes, Augusto Batagin-Neto, and Paulo N. Lisboa-Filho. Material preparation, data collection and analysis were performed by Orisson P. Gomes, João P. C. Rheinheimer, and Leonardo F. G. Dias. The first draft of the manuscript was written by Orisson P. Gomes and all authors commented and revised it critically for important intellectual content on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gomes, O.P., Rheinheimer, J.P.C., Dias, L.F.G. et al. Revisiting the hydroxylation phenomenon of SiO2: a study through “hard-hard” and “soft–soft” interactions. J Mol Model 28, 115 (2022). https://doi.org/10.1007/s00894-022-05107-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05107-w