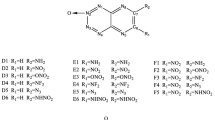
Abstract
A series of nitro-imidazole derivatives were designed by replacing hydrogen atoms on imidazole ring with nitro group one by one. In order to investigate the thermodynamic stability, heat of formation (HOF), and bond dissociation energy (BDE) are calculated at the B3PW91/6-311+G(d,p) level. In order to investigate the impact sensitivity and detonation property, the drop height (H50), free space per molecule in crystal lattice (ΔV), detonation velocity (D), and detonation pressure (P) are calculated by using the empirical Kamlet–Jacobs (K-J) equation. The results show that the thermal stabilities of title molecules are determined by whether nitro group is associated to 1-position or not and accompanied with the steric hindrance between nitro groups and the charge population on the carbon atoms of imidazole ring. The excellent impact sensitivity and detonation performance of title molecules are also evaluated. On the consideration both of stability and detonation characters, 2,4,5-trinitro-1H-imidazole (D = 8.98 km/s, P = 36.70 GPa) is screened out as the potential high-energy-density molecule for further research.

Similar content being viewed by others
References
Ni K-K, Ospelkaus S, De Miranda M, Pe’er A, Neyenhuis B, Zirbel J, Kotochigova S, Julienne P, Jin D, Ye J (2008) A high phase-space-density gas of polar molecules. Science 322:231–235
Ross M, Ree F (1980) Repulsive forces of simple molecules and mixtures at high density and temperature. J Chem Phys 73:6146–6152
Mondal T, Saritha B, Ghanta S, Roy T, Mahapatra S, Prasad MD (2009) On some strategies to design new high energy density molecules. J Mol Struct THEOCHEM 897:42–47
Wang Y, Liu Y, Song S, Yang Z, Qi X, Wang K, Liu Y, Zhang Q, Tian Y (2018) Accelerating the discovery of insensitive high-energy-density materials by a materials genome approach. Nat Commun 9:2444
Liu T, JIa J, Li B, Gao K (2019) Theoretical exploration on structural stabilities and detonation properties of nitrimino substituted derivatives of cyclopropane. Chin J Struct Chem 38:688–694
Li B, Zhou M, Peng J, Li L, Guo Y (2019) Theoretical calculations about nitro-substituted pyridine as high-energy-density compounds (HEDCs). J Mol Model 25:23
Flüescheim B, Holmes EL (1928) CCCXCIX.—pentanitroaniline. J Chem Soc:3041–3046
Boddu VM, Viswanath DS, Ghosh TK, Damavarapu R (2010) 2,4,6-Triamino-1,3,5-trinitrobenzene (TATB) and TATB-based formulations—a review. J Hazard Mater 181:1–8
Thottempudi V, Forohor F, Parrish DA, Shreeve JM (2012) Tris (triazolo) benzene and its derivatives: high-density energetic materials. Angew Chem Int Ed 51:9881–9885
Zhang Y, Parrish DA, Jean'ne MS (2013) Derivatives of 5-nitro-1, 2, 3-2H-triazole–high performance energetic materials. J Mater Chem A 1:585–593
Cooper PW (2018) Explosives engineering. Wiley
Bulusu S, Damavarapu R, Autera JR, Behrens R, Minier LM, Villanueva J, Jayasuriya K, Axenrod T (1995) Thermal rearrangement of 1,4-dinitroimidazole to 2,4-dinitroimidazole: characterization and investigation of the mechanism by mass spectrometry and isotope labeling. J Phys Chem 99:5009–5015
Bracuti A (1995) Crystal structure of 2, 4-dinitroimidazole (24DNI). J Chem Crystallogr 25:625–627
Ravi P (2017) Experimental study and ab-initio calculations on the molecular structure, infrared and Raman spectral properties of dinitroimidazoles. Chem Data Collect 9-10:11–23
Grimmett MR, Hua S-T, Chang K-C, Foley S, Simpson J (1989) 1, 4-Dinitroimidazole and derivatives. Structure and thermal rearrangement. Aust J Chem 42:1281–1289
Minier L, Behrens R, Bulusu S (1996) Mass spectra of 2, 4-dinitroimidazole and its isotopomers using simultaneous thermogravimetric modulated beam mass spectrometry. J Mass Spectrom 31:25–30
De Bondt H, Ragia E, Blaton N, Peeters O, De Ranter C (1993) Structure of 4 (5)-nitroimidazole at 100 K. Acta Crystallogr Sect C Cryst Struct Commun 49:693–695
Carvalho TMT, Amaral LMPF, Morais VMF, Ribeiro da Silva MDMC (2017) Calorimetric and computational studies for three nitroimidazole isomers. J Chem Thermodyn (105):267–275
Windler GK, Scott BL, Tomson NC, Leonard PW (2015) Crystal structure of 4,5-di-nitro-1H-imidazole. Acta Crystallogr Sect E Cryst Commun 71:o634
Coburn MD (1977) Ammonium 2, 4, 5-trinitroimidazole, in, Google Patents
Cho SG, Cho JR, Goh EM, Kim JK, Damavarapu R, Surapaneni R (2005) Synthesis and characterization of 4, 4′, 5, 5′-tetranitro-2, 2′-bi-1H-imidazole (TNBI). Propellants Explos Pyrotech 30:445–449
Damavarapu R, Jayasuriya K, Vladimiroff T, Iyer S (1995) 2, 4-dinitroimidazole-a less sensitive explosive and propellant made by thermal rearrangement of molten 1, 4 dinitroimidazole, in, Google Patents
Cho JR, Kim KJ, Cho SG, Kim JK (2002) Synthesis and characterization of 1-methyl-2,4,5-trinitroimidazole (MTNI). J Heterocyclic Chem 39:141–147
Duddu R, Dave PR, Damavarapu R, Gelber N, Parrish D (2010) Synthesis of N-amino- and N-nitramino-nitroimidazoles. Tetrahedron Lett 51:399–401
Windaus A, Vogt W (1907) Synthese des Imidazolyl-äthylamins. Ber Dtsch Chem Ges 40:3691–3695
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1
Kamlet MJ, Ablard JE (1968) Chemistry of detonations. II. Buffered equilibria. J Chem Phys 48:36–42
Kamlet MJ, Jacobs SJ (1968) Chemistry of detonations. I. a simple method for calculating detonation properties of C–H–N–O explosives. J Chem Phys 48:23–35
Talawar M, Sivabalan R, Mukundan T, Muthurajan H, Sikder A, Gandhe B, Rao AS (2009) Environmentally compatible next generation green energetic materials (GEMs). J Hazard Mater 161:589–607
Politzer P, Murray JS (2016) High performance, low sensitivity: conflicting or compatible? Propellants Explos Pyrotechnics 41:414–425
Pospíšil M, Vávra P, Concha MC, Murray JS, Politzer P (2010) A possible crystal volume factor in the impact sensitivities of some energetic compounds. J Mol Model 16:895–901
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbé A (2009) An electrostatic interaction correction for improved crystal density prediction. Mol Phys 107:2095–2101
Politzer P, Martinez J, Murray JS, Concha MC (2010) An electrostatic correction for improved crystal density predictions of energetic ionic compounds. Mol Phys 108:1391–1396
Politzer P, Murray JS (2015) Some molecular/crystalline factors that affect the sensitivities of energetic materials: molecular surface electrostatic potentials, lattice free space and maximum heat of detonation per unit volume. J Mol Model 21:25
An CW, Guo XD, Song XL, Wang Y, Li FS (2009) Preparation and safety of well-dispersed RDX particles coated with cured HTPB. J Energ Mater 27:118–132
Politzer P, Murray JS (2015) Perspectives on the crystal densities and packing coefficients of explosive compounds. Struct Chem 27:401–408
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, B., Li, L. & Chen, S. Thermal stability and detonation character of nitro-substituted derivatives of imidazole. J Mol Model 25, 298 (2019). https://doi.org/10.1007/s00894-019-4190-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4190-5