Abstract
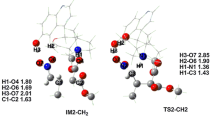
The mechanism of the copper(I)-catalyzed cyclopropanation reaction for methyl diazoacetate with both (Z)- and (E)-but-2-ene stereoisomers has been studied using the 6-311++G(d,p) basis set by means of M06-2X and O3LYP functionals. According to both methods, the rate-limiting step is the formation of a copper-carbene intermediate, formed by association between methyl diazoacetate and bis(acetonitrile)-copper(I) ion with the concomitant extrusion of dinitrogen. Cis/trans diastereoselectivity for the cyclopropanation reaction of a 1,2-disubstituted alkene ((Z)-but-2-ene) has been theoretically studied for the first time through the proper location of transition states on the potential-energy surface with the O3LYP method, since no transition structures could be found with the M06-2X functional due to the extreme flatness of the potential-energy surface. The calculated stereoselectivities involving two acetonitrile ligands or one dichloromethane molecule show qualitative agreement with experimental data. This study allows attributing the origin of the selectivity to steric interactions between the ligands of the catalyst system and the olefin substituents. The comparison between the corresponding activation barriers for the direct insertion step shows a higher reactivity for the Z stereoisomer of but-2-ene, consistently with the larger reactant destabilization through steric interactions.




Similar content being viewed by others
References
Salaün J (2000) Top Curr Chem 207:1–67. https://doi.org/10.1007/3-540-48255-5_1
Kulinkovich OG (2015) Cyclopropanes in organic synthesis. Wiley, Hoboken. https://doi.org/10.1002/9781118978429
Lebel H, Marcoux J-F, Molinaro C, Charette AB (2003) Chem Rev 103:977–1050. https://doi.org/10.1021/cr010007e
Pellissier H (2008) Tetrahedron 64:7041–7095. https://doi.org/10.1016/j.tet.2008.04.079
Bartoli G, Bencivenni G, Dalpozzo R (2014) Synthesis 46:979–1029. https://doi.org/10.1055/s-0033-1340838
García JI, Salvatella L, Pires E, Fraile JM, Mayoral JA (2014) In: Knochel A, Holander GA (eds) Comprehensive organic synthesis II. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-08-097742-3.00426-2
Charette AB, Lebel H, Roy M-N (2014) In: Alexakis A, Krause N, Woodward S (eds) Copper-catalyzed asymmetric synthesis. Wiley-VCH, Weinheim. https://doi.org/10.1002/9783527664573.ch8
Besora M, Braga AAC, Sameera WMC, Urbano J, Fructos MR, Pérez PJ, Maseras F (2015) J Organomet Chem 784:2–12. https://doi.org/10.1016/j.jorganchem.2014.10.009
Salomon RG, Kochi JK (1973) J Am Chem Soc 95:3300–3310. https://doi.org/10.1021/ja00791a038
Díaz-Requejo MM, Belderrain TR, Nicasio MC, Prieto F, Pérez PJ (1999) Organometallics 18:2601–2609. https://doi.org/10.1021/om990270u
Fraile JM, García JI, Martínez-Merino V, Mayoral JA, Salvatella L (2001) J Am Chem Soc 123:7616–7625. https://doi.org/10.1021/ja003695c
Fraile JM, García JI, Gissibl A, Mayoral JA, Pires E, Reiser O, Roldán M, Villalba I (2007) Chem Eur J 13:8830–8839. https://doi.org/10.1002/chem.200700681
Straub BF, Gruber I, Rominger F, Hofmann P (2003) J Organomet Chem 684:124–143. https://doi.org/10.1016/S0022-328X(03)00520-5
Özen C, Tüzün NŞ (2008) Organometallics 27:4600–4610. https://doi.org/10.1021/om800094k
Meng Q, Li M, Tang D, Shen W, Zhang J (2004) J Mol Struct (THEOCHEM) 711:193–199. https://doi.org/10.1016/j.theochem.2004.06.050
Drudis-Solé G, Maseras F, Lledós A, Vallribera A, Moreno-Mañas M (2008) Eur J Org Chem 2008:5614–5621. https://doi.org/10.1002/ejoc.200800762
Rasmussen T, Jensen JF, Østergaard N, Tanner D, Ziegler T, Norrby P-O (2002) Chem Eur J 8:177–184. https://doi.org/10.1002/1521-3765(20020104)8:1<177::AID-CHEM177>3.0.CO;2-H
García JI, Jiménez-Osés G, Mayoral JA (2011) Chem Eur J 17:529–539. https://doi.org/10.1002/chem.201001262
Cohen AJ, Handy NC (2001) Mol Phys 99:607–615. https://doi.org/10.1080/00268970010023435
Handy NC, Cohen AJ (2001) Mol Phys 99:403–412. https://doi.org/10.1080/00268970010018431
Fraile JM, García JI, Herrerías CI, Pires E (2017) Synthesis 49:1444–1460. https://doi.org/10.1055/s-0036-1588699
Angulo B, Fraile JM, Herrerías CI, Mayoral JA (2017) RSC Adv 7:19417–19424. https://doi.org/10.1039/c7ra01017f
Liang HC, Kim E, Incarvito CD, Rheingold AL, Karlin KD (2002) Inorg Chem 41:2209–2212. https://doi.org/10.1021/ic010816g
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A.02. Gaussian, Wallingford
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09, revision D.01. Gaussian, Wallingford
Zhao Y, Truhlar DG (2008) Theor Chem Accounts 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Bozic-Weber B, Chaurin V, Constable EC, Housecroft CE, Meuwly M, Neuburger M, Rudd JA, Schönhofer E, Siegfried L (2012) Dalton Trans 41:14157–14169. https://doi.org/10.1039/C2DT31159C
Tamasi G, Bonechi C, Rossi C, Cini R, Magnani A (2016) J Coord Chem 69:404–424. https://doi.org/10.1080/00958972.2015.1132416
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523–5527. https://doi.org/10.1021/j100377a021
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154–2161. https://doi.org/10.1063/1.456010
Laury ML, Carlson MJ, Wilson AK (2012) J Comput Chem 33:2380–2387. https://doi.org/10.1002/jcc.23073
Hu S-Z, Zhou Z-H, Xie Z-X, Robertson BE (2014) Z Krist 229:517–523. https://doi.org/10.1515/zkri-2014-1726
Stojanović M, Aleksić J, Baranac-Stojanović M (2015) Tetrahedron 71:5119–5123. https://doi.org/10.1016/j.tet.2015.06.22
Leung BO, Reid DL, Armstrong DA, Rauk A (2004) J Phys Chem A 108:2720–2725. https://doi.org/10.1021/jp030265a
Acknowledgments
The Instituto de Síntesis Química y Catálisis Homogénea (ISQCH) and the Instituto de Biocomputación y Física de Sistemas Complejos (BIFI) (Consejo Superior de Investigaciones Científicas (CSIC)–Universidad de Zaragoza) are thanked for the allocation of computer time. Financial support from Ministerio de Economía y Competitividad (MINECO) (Project CTQ2014-52367-R), Gobierno de Aragón, European Regional Development Fund (Consolidated Group E11), and European Social Fund is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 104 kb)
Rights and permissions
About this article
Cite this article
Angulo, B., Herrerías, C.I., Hormigón, Z. et al. Copper-catalyzed cyclopropanation reaction of but-2-ene. J Mol Model 24, 195 (2018). https://doi.org/10.1007/s00894-018-3737-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3737-1