Abstract
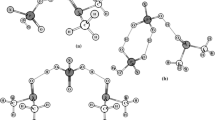
The molecular structures and H-bonding interactions in the phosphinic acid dimer and the complex of phosphinic acid with N,N-dimethylformamide (DMF) were investigated by density functional theory calculations at the B3LYP level of theory. In order to better understand these phenomena, the individual molecules were also studied. The results were compared with previously obtained data for similar H-bonded complexes of phosphoric and phosphorous acids with DMF. Various correlations were found between geometric characteristics and parameters derived from Bader’s theory and natural bond orbital analysis.

Irina V. Fedorova and Lyubov P. Safonova.
Ab initio study of hydrogen bonding in the H3PO2 dimer and H3PO2–DMF complex.
The acids of phosphorus are considered suitable candidates for ionomers due to their efficient proton transport properties. In order to better understand their molecular structures and their H-bond characteristics in real liquids, the most stable configurations of the H3PO2 dimer and the H3PO2–DMF complex were examined using computational methods in quantum chemistry. It was found that the strength of the H-bonding interactions in these systems depends strongly on the environment



Similar content being viewed by others
References
Ewig CS, Van Wazer JR (1985) J. Am. Chem. Soc. 107:1965–1971
Gonzalez L, Mo O, Yanez М, Elguero J (1998) J. Chem. Phys. 109:2685–2693
Range K, McGrath MJ, Lopez X, York D (2004) J. Am. Chem. Soc. 126:1654–1665
Heggen B, Roy S, Müller-Plathe F (2008) J. Phys. Chem. 112:14209–14215
Ahmadi I, Rahemi H, Tayyari SF (2005) J. Korean Chem. Soc. 49:129–137
Joswig J-O, Hazebroucq S, Seifert G (2007) J. Mol. Struct. THEOCHEM 816:119–123
Yekutiel M, Lane JR, Gupta P, Kjaergaard HG (2010) J. Phys. Chem. A 114:7544–7552
Stefan T, Janoschek R (2000) J. Mol. Model. 6:282–288
Korbridge DEC (1985) Phosphorus—an outline of its chemistry, biochemistry and technology, 3rd edn. Elsevier, Amsterdam
Zhao TS, Kreuer K-D, Nguyen TV (2007) Advances in fuel cells, vol I. Elsevier, Oxford
Dippel T, Kreuer KD, Lassègues JC, Rodriguez D (1993) Solid State Ionics 61:41–46
Blessing RH (1988) Acta Cryst. B44:334–340
Becker G, Hausen HD, Mundt O, Schwarz W, Wagner CT, Vogt T (1990) Z. Anorg. Allg. Chem. 591:17–31
Iwadate Y, Tani Y, Fukushima K, Misawa M, Fukunaga T, Itoh K, Nakazawa T (2005) J Neutron Res. 13:139–144
Williams JM, Peterson SW (1971) Spectrosc. Inorg. Chem. 2:1–56
Detoni S, Hadzi D, Orel B (1976) J. Mol. Struct. 33:279–288
Tsuchida E (2006) J. Phys. Soc. Jpn. 75:054801(5)
Tromp R, Spieser S, Neilson G (1999) J. Chem. Phys. 110:2145–2150
Vilciauskas L, Tuckerman ME, Melchior JP, Bester G, Kreuer KD (2013) Solid State Ionics 252:34–39
Fadeeva JA, Safonova LP, Persson I (2010) Phys. Chem. Chem. Phys. 12:8977–8984
Li S, Fried JR (2013) Macromol. Theory Simul. 22:410–425
Shirata K, Kawauchi S (2015) J. Phys. Chem. B 119:592–603
Khatuntseva EA, Krest’yaninov MA, Fedorova IV, Kiselev MG, Safonova LP (2015) Russ. J. Phys. Chem. A 89:2248–2253
Fedorova IV, Krestyaninov MA, Kiselev MG, Safonova LP (2016) J. Mol. Struct. 1106:424–429
Fedorova IV, Khatuntseva EA, Krest’yaninov MA, Safonova LP (2016) Russ. J. Phys. Chem. A 90:293–299
Steiner T (2002) Angew. Chem. Int. Ed. 41:48–76
Turi L (1996) J. Phys. Chem. 100:11285–11291
Qian W, Krimm S (2001) J. Phys. Chem. A 105:5046–5053
Wilson CC, Morrison CA (2002) Chem. Phys. Lett. 362:85–89
Krawietz TR, Lin P, Lotterhos KE, Torres PD, Barich DH, Clearfield A, Haw JF (1998) J. Am. Chem. Soc. 120:8502–8511
Zhang D, Yan L (2010) J. Phys. Chem. B 114:12234–12241
Ilczyszyn MM (2002) J. Mol. Struct. 611:119–129
Fedorova IV, Safonova LP (2016) Struct. Chem. 27:1189–1198
Scheiner AC, Baker J, Andzelm JW (1997) J. Comput. Chem. 18:775–795
Sharma A, Ohanessian G, Clavaguéra C (2014) J. Mol. Model. 20:2426–2434
Vilciauskas L, Paddison SJ, Kreuer K-D (2009) J. Phys. Chem. A 113:9193–9201
Krest’yaninov MA, Kiselev MG, Safonova LP (2015) Russ. J. Phys. Chem. A 89:608–615
Weinhold F (1997) J. Mol. Struct. THEOCHEM 398-399:181–197
Koch U, Popelier PLA (1995) J. Chem. Phys. 99:9747–9754
Popelier PLA (1998) J. Phys. Chem. A 102:1873–1878
Cossi M, Rega N, Scalmani G, Barone V (2003) J. Comput. Chem. 24:669–681
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision a.01. Gaussian, Inc., Wallingford
Sousa CF, Fernandes PA, Ramos MJ (2007) J. Phys. Chem. A 111:10439–10452
Lee C, Yang W, Parr RG (1988) Phys. Rev. B 37:785–789
Becke AD (1993) J. Chem. Phys. 98:5648–5652
Ditchfield R, Hehre WJ, Pople JA (1971) J. Chem. Phys. 54:724–728
Safonova LP, Kiselev MG, Fedorova IV (2013) Pure Appl. Chem. 85:225–236
van Duijneveldt FB, van Duijneveldt-van de Rijdt JGCM, van Lenthe JH (1994) Chem. Rev. 94:1873–1885
Boys S, Bernardi F (2002) Mol. Phys. 19:553–566
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Keith TA (2010) AIMAll, version 10.05.04. aim.tkgristmill.com
Espinosa E, Molins E, Lecomte C (1998) J Chem. Phys. Lett. 285:170–173
Weinhold F, Landis CR (2005) Valency and bonding. A natural bond orbital donor–acceptor perspective. Cambridge University Press, Cambridge
Williams JM (1966) Ph.D. thesis. Washington State University
Schultz G, Hargittai I (1993) J. Phys. Chem. 97:4966–4969
Acknowledgments
This work was partially supported by the Russian Foundation for Basic Research (no. 15-43-03088).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Table S1 shows the intramolecular distances for phosphoric, phosphorous, and phosphinic acids calculated at different levels of theory, and a comparison of those distances with the corresponding values obtained experimentally. Table S2 lists the frequency assignments for the H3PO2 monomer calculated with different methods, and compares them with the corresponding experimental data.
ESM 1
(DOC 54.5 kb)
Rights and permissions
About this article
Cite this article
Fedorova, I.V., Safonova, L.P. Ab initio study of hydrogen bonding in the H3PO2 dimer and H3PO2–DMF complex. J Mol Model 23, 220 (2017). https://doi.org/10.1007/s00894-017-3393-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3393-x