Abstract
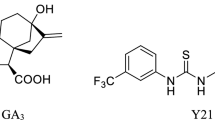
N-substituted phthalimides (NSPs) that show multiple gibberellin (GA)-like effects on the growth and development of higher plants have been reported. These NSPs may represent a potential alternative to commercial GAs. Therefore, in this work, molecular docking and molecular dynamics simulations were used to explore the mode of interaction between some NSPs and the GA receptor GID1A in order to clarify the relationship between structure and GA-like activity in the NSPs. The results obtained demonstrate that both a multiple-hydrogen-bond network and a “hat-shaped” hydrophobic interaction play important roles in the binding of the NSPs to GID1A. The carbonyl group of a phthalimide fragment in the NSPs acted in a similar manner to the pharmacophore group 6-COOH in GAs, forming multiple-hydrogen-bond interactions with residues Ser191 and Tyr322 in the binding domain of GID1A. Comparative molecular field analysis (CoMFA) and comparative molecular similarity indices analysis (CoMSIA) were used to further study the 3D quantitative structure–activity relationship (3D-QSAR) of the NSPs. It was confirmed that the GA-like activity of these NSPs is strongly linked to a few H-bond donor and acceptor field contributions of the NSPs to the H-bond interactions with GID1A. Five new NSP molecules D1–D5 were designed using the binding domain of GID1A and then docked into the receptor. D1 and D4 were shown to have good docking scores due to enhanced hydrophobic contact. We hope that these results will provide useful guidance in the rational design of new NSPs.

Molecular docking and molecular dynamics simulations were used to explore the interaction mode between N-substituted phthalimides (NSPs) and the gibberellin (GA) receptor GID1A to clarify the relationship between structure and GA-like activity for the NSPs. A multiple-hydrogen-bond network and a “hat-shaped” hydrophobic interaction were found to play important roles in the binding of NSPs to GID1A. These H-bond interactions were further shown to influence the GA-like activity of the NSPs via some H-bond donor and acceptor molecular fields



Similar content being viewed by others
References
Davies PJ (1995) Plant hormones: physiology, biochemistry and molecular biology. Kluwer, Dordrecht, p 64
Sun TP (2011) Curr Biol 21:338–345
Suttle JC (1983) News Bull Br Plant Growth Regul Grp 6:11–19
Suttle JC, Schreiner DR (1982) J Plant Growth Regul 1:139–146
Los M, Kust CA, Lamb G, Diehl RE (1980) HortSci 15:22–23
Suttle JC, Hultstrand JF (1987) Plant Physiol 84:1068–1073
Simonovic A, Grubisic D, Giba Z, Konjevic R (2000) J Plant Growth Regul 32:91–97
Yalpani N, Suttle JC, Hultstrand JF, Rodaway SJ (1989) Plant Physiol 91:823–828
Choma ME, Himelrick DG (1984) Sci Hortic 22:257–264
Li WQ, Liu XJ, Yamaguchi S (2005) Plant Physiol Commun 41:111–115 (in Chinese)
Zhang GH, Zhang YJ, Cong RC, Dong KQ, Gu RZ (2009) Acta Bot Boreali-Occidentalia Sin 29:412–419 (in Chinese)
Stoddart JL (1979) Planta 146:353–361
Keith B, Foster NA, Bonettemaker M, Srivastava LM (1981) Plant Physiol 68:344–348
Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow T, Hsing YC, Kitano H, Yamaguchi I, Matsuoka M (2005) Nature 437:693–698
Nakajima M, Shimada A, Takashi Y, Kim YC, Park SH, Ueguchi-Tanaka M, Suzuki H, Karoh E, Iuchi S, Kobayashi M, Maeda T, Matsuoka M, Yamaguchi I (2006) Plant Cell 46:880–889
Murase K, Hirano Y, Sun TP, Hakoshima T (2008) Nature 456:459–464
Shimada A, Ueguchi-Tanaka M, Nakatsu T, Nakajima M, Naoe Y, Ohmiya H, Kato H, Matsuoka M (2008) Nature 456:520–524
Duan HX, Li DL, Liu HC, Liang DS, Yang XL (2013) J Mol Model 19:4613–4624
RCSB (2015) Protein Data Bank. http://www.rcsb.org/pdb/home/home.do
Tripos Associates (2006) Sybyl 7.3. Tripos Associates, St. Louis
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T Jr, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Comperts R, Stratmann RE, Yazyey O, Austin SJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JC, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, revision A.1. Gaussian, Inc., Pittsburgh
Jain AN (1996) J Comput Aided Mol Des 10:427–440
Welch W, Ruppert J, Jain AN (1996) Chem Biol 3:449–462
Clark RD, Strizhev A, Leonard JM, Blake JF, Matthew JB (2002) J Mol Graph Model 20:281–295
Hess B, Kutzner C, van der Spoel D, Lindahl E (2008) J Chem Theory Comput 4:435–447
Berendsen HJC, van der Spoel D, van Drunen R (1995) Comput Phys Commun 91:43–56
Schüttelkopf AW, van Aalten DMF (2004) Acta Crystallogr D 60:1355–1363
Hess B, Bekker H, Berendsen HJC, Fraajie JGEM (1997) J Comput Chem 18:1463–1472
Essman U, Perela L, Berkowitz ML, Darden T, Lee H, Pedersen LG (1995) J Chem Phys 103:8577–8592
Golbraikh A, Tropsha A (2002) J Mol Graph Model 20:269–276
Xiang HY, Takeuchi H, Tsunoda Y, Nakajima M, Murata K, Ueguchi Tanaka M, Kidokoro S, Kezuka Y, Nonaka T, Matsuoka M, Katoh E (2011) J Mol Recognit 24:275–282
Ueguchi Tanaka M, Nakajima M, Katoh E, Ohmiya H, Asano K, Saji S, Xiang HY, Ashikari M, Kitano H, Yamaguchi I, Matsuoka M (2007) Plant Cell 19:2140–2155
Acknowledgments
This work was supported by the Chinese Universities Scientific Fund (2012QJ024, 2013QJ001), the National Scientific and Technology Supporting Program of China (2011BAE06B05-5), and the National Natural Science Foundation of China (31101109).
Author contributions
Conceived and designed the experiments: D.L. Li, H.X. Duan; analyzed the data: D.L. Li, H.X. Duan; wrote the first draft of the manuscript: D.L. Li, S.Q. Du; made critical revisions and approved the final version: H.X. Duan, W.M. Tan. All authors reviewed and approved the final manuscript.
Ethical statement
I, the corresponding author, have full control of all primary data and I agree to allow the journal to review our data if requested. I have no financial relationship with the organization that sponsored the research and authorship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 557 kb)
Rights and permissions
About this article
Cite this article
Li, D., Du, S., Tan, W. et al. Computational insight into the structure–activity relationship of novel N-substituted phthalimides with gibberellin-like activity. J Mol Model 21, 271 (2015). https://doi.org/10.1007/s00894-015-2817-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2817-8