Abstract
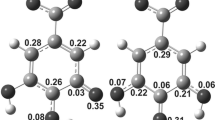
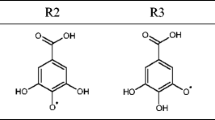
The M05-2X/6-311++G(d,p) and B3LYP-D2/6-311++G(d,p) models are used to evaluate scavenging potency of gallic acid. The hydrogen atom transfer (HAT), sequential proton loss electron transfer (SPLET), and single electron transfer followed by proton transfer (SET-PT) mechanisms of gallic acid with some radicals (•OO−, •OH, and CH3OO•) were investigated using the corresponding thermodynamic quantities: bond dissociation enthalpy (BDE), ionization potential (IP), and proton affinity (PA). Namely, the ΔHBDE, ΔHIP, and ΔHPA values of the corresponding reactions in some solvents (water, DMSO, pentylethanoate, and benzene) are investigated using an implicit solvation model (SMD). An approach based on the reactions enthalpies related to the examined mechanisms is applied. This approach shows that a thermodynamically favored mechanism depends on the polarity of reaction media and properties of free radical reactive species. The most acidic 4-OH group of gallic acid is the active site for radical inactivation. The results of this investigation indicate that the SPLET mechanism can be a favored reaction pathway for all three radicals in all solvents, except for •OH in the aqueous solution. In water, gallic acid can inactivate •OH by the HAT mechanism.

Influence of different solvents on antioxidative mechanisms of gallic acid



Similar content being viewed by others
References
Bianco M-A, Handaji A, Savolainen H (1998) Quantitative analysis of ellagic acid in hardwood samples. Sci Total Environ 222:123–126
Pettersen RC, Ward JC, Lawrence AH (1993) Detection of northern red oak wetwood by fast heating and ion mobility spectrometric analysis. Holzforschung 47:513–522
Koga T, Moro K, Nakamori K, Yamakoshi J, Hosoyama H, Kataoka S, Ariga T (1999) Increase of antioxidative potential of rat plasma by oral administration of proanthocyanidin-rich extract from grape seeds. J Agric Food Chem 47:1892–1897
Burns J, Gardner PT, O’Neil J, Crawford S, Morecroft I, McPhail DB, Lister C, Matthews D, MacLean MR, Lean MEJ, Duthie GG, Crozier A (2000) Relantionship among antioxidant activity, vasodilation capacity, and phenolic content of red wines. J Agric Food Chem 48:220–230
Sakagami H, Yokote Y, Akahane K (2001) Changes in amino acid pool and utilization during apoptosis in HL-60 cells induced by epigallocatechin gallate or gallic acid. Anticancer Res 21:2441–2447
Krilov A, Holmgren A, Gref R, Öhman L-O (1993) Effect of gallic acid on metals: an FT-IR study of complexes between gallic acid and sawblade steel. Holzforschung 47:239–246
Shukla YN, Srivastava A, Kumar S, Kumar S (1999) Phytotoxic and antimicrobial constituents of Argyreia speciosa and Oenothera biennis. J Ethnopharmacol 67:241–245
Gutteridge JMC, Halliwell B (1994) Antioxidants in nutrition, health, and disease, 1st edn. Oxford University Press, Oxford
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Klein E, Lukeš V, Ilčin M (2007) DFT/B3LYP study of tocopherols and chromans antioxidant action energetics. Chem Phys 336:51–57
Litwinienko G, Ingold KU (2007) Solvent effects on the rates and mechanisms of reaction of phenols with free radicals. Acc Chem Res 40:222–230
Di Meo F, Lemaur V, Cornil J, Lazzaroni R, Duroux J-L, Olivier Y, Trouillas P (2013) Free radical scavenging by natural polyphenols: atom versus electron transfer. J Phys Chem A 117:2082–2092
Foti MC, Daquino C, Geraci C (2004) Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH• radical in alcoholic solutions. J Org Chem 69:2309–2314
Marković Z, Milenković D, Đorović J, Jeremić S (2013) Solvation enthalpies of the proton and electron in polar and non-polar solvents. J Serb Soc Comput Mech 7:1–9
Bartmess JE (1994) Thermodynamics of the electron and the proton. J Phys Chem 98:6420–6424
Zhao Y, Schultz NE, Truhlar DG (2005) Exchange-correlation functional with broad accuracy for metallic and nonmetallic compounds, kinetics, and noncovalent interactions. J Chem Phys 123(1–4):161103
McLean AD, Chandler GS (1980) Contracted gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J Chem Phys 72:5639–5648
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick AD, Rabuck KD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, HeadGordon M, Replogle ES, Pople JA (2010) Gaussian 09, revision C.01. Gaussian Inc, Wallingford
Black G, Simmie JM (2010) Barrier heights for H-atom abstraction by HO2 from n-butanol-a simple yet exacting test for model chemistries? J Comput Chem 31:1236–1248
Galano A, Macias-Ruvalcaba NA, Medina-Campos ON, Pedraza-Chaverri J (2010) Mechanism of the OH radical scavenging activity of nordihydroguaiaretic acid: a combined theoretical and experimental study. J Phys Chem B 114:6625–6635
Marković ZS, Dimitrić Marković JM, Doličanin ĆB (2010) Mechanistic pathways for the reaction of quercetin with hydroperoxy radical. Theor Chem Accounts 127:69–80
Zavala-Oseguera C, Alvarez-Idaboy JR, Merino G, Galano A (2009) OH radical gas phase reactions with aliphatic ethers: a variational transition state theory study. J Phys Chem A 113:13913–13920
Alberto ME, Russo N, Grand A, Galano A (2013) A physicochemical examination of the free radical scavenging activity of Trolox: mechanism, kinetics and influence of the environment. Phys Chem Chem Phys 15:4642–4650
Marković Z, Milenković D, Đorović J, Dimitrić Marković JM, Stepanić V, Lučić B, Amić D (2012) PM6 and DFT study of free radical scavenging activity of morin. Food Chem 134:1754–1760
Grimme S (2011) Density functional theory with London dispersion corrections. WIREs Comput Mol Sci 1:211–228
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion coorrection. J Comput Chem 27:1787–1799
Bayach I, Sancho-García JC, Di Meo F, Weber J-FF, Trouillas P (2013) π-stacked polyphenolic dimers: a case study using dispersion-corrected methods. Chem Phys Lett 578:120–125
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Carpenter JE, Weinhold F (1988) Analysis of the geometry of the hydroxymethyl radical by the “different hybrids for different spins” natural bond orbital procedure. J Mol Struct (Theochem) 169:41–62
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Medina ME, Iuga C, Alvarez-Idaboy JR (2013) Antioxidant activity of propyl gallate in aqueous and lipid media: a theoretical study. Phys Chem Chem Phys 15:13137–13146
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
Nsangou M, Dhaouadi Z, Jaidane N, Ben Lakhdar Z (2008) DFT study of the structure of hydroxybenzoic acids and their reactions with •OH and •O2 − radicals. J Mol Struct (Theochem) 850:135–143
Leopoldini M, Marino T, Russo N, Toscano M (2004) Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J Phys Chem A 108:4916–4922
Marković ZS, Dimitrić-Marković JM, Milenković D, Filipović N (2011) Structural and electronic features of baicalein and its radicals. Monatsh Chem 142:145–152
Rimarčik J, Lukeš V, Klein E, Ilčin M (2010) Study of the solvent effect on the enthalpies of homolytic and heterolytic N−H bond cleavage in p-phenylenediamine and tetracyano-p-phenylenediamine. J Mol Struct (Theochem) 952:25–30
Vaganek A, Rimarčik J, Lukeš V, Klein E (2012) On the energetics of homolytic and heterolytic O–H bond cleavage in flavonoids. Comp Theor Chem 991:192–200
Shahidi F, Janitha PK, Wanasundara PD (1992) Phenolic antioxidants. Crit Rev Food Sci Nutr 32:67–103
Hussein MA (2011) A convenient mechanism for the free radical scavenging activity of resveratrol. Int J Phytomed 3:459–469
Fang Y-Z, Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition 18:872–879
Acknowledgments
The authors gratefully acknowledge financial support from the Ministry of Science of the Republic of Serbia (Projects No. 172015 and 174028), and the Ministry of Science, Education and Sports of the Republic of Croatia (Projects Nos.: 079-0000000-3211 and 098-0982464-2511).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 2934 kb)
Rights and permissions
About this article
Cite this article
Đorović, J., Marković, J.M.D., Stepanić, V. et al. Influence of different free radicals on scavenging potency of gallic acid. J Mol Model 20, 2345 (2014). https://doi.org/10.1007/s00894-014-2345-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2345-y