Abstract
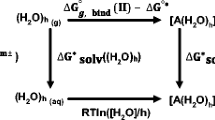
A relative complete study on the mechanisms of the proton transfer reactions of 2-thioxanthine was carried out with density functional theory. The models were designed with monohydrated and dihydrated microsolvent catalyses either with or without the presence of water solvent considered with the polarized continuum model (PCM). A total number of 114 complexes and 67 transition states were found with the B3LYP/6-311+G** calculations. The energies were refined with both B3LYP/aug-cc-pVTZ and PCM-B3LYP/aug-cc-pVTZ methods. The activation energies were reported with respect to the Gibbs free energies obtained in conjunction with the standard statistical thermodynamics. Possible reaction pathways were confirmed with the intrinsic reaction coordinates. Pathways via C8 atom on the imidazole ring, via the bridged C4 and C5 atoms between pyrimidine and imidazole rings and via N, O and S atom on the pyrimidine ring were examined. The results show that the most feasible pathway is the proton transfers within the long range solvent surrounding via the N, O and S atoms in the pyrimidine ring with di-hydrated catalysis: N(7)H + 2H2O → IM89 → IM90 → P13 + 2H2O → IM91 → IM92 → P6 + 2H2O → IM71 → IM72 → P7 + 2H2O → IM107 → IM108 → P18 + 2H2O → IM111 → IM112 → P19 + 2H2O → IM113 → IM114 → P17 + 2H2O → IM105 → IM106 → N(9)H + 2H2O that has the highest energy barrier of 44.0 kJ mol−1 in the transition of IM89 to IM90 via TS54. The small energy barrier is in good agreement with the experimental observation that 2-TX tautomerizes at room temperature in water. In the aqueous phase, the most stable intermediate is found to be IM21 [N(7)H + 2H2O] and the possible co-existing species are the monohydrated IM1, IM9, IM39 and IM46, and the di-hydrated IM5, IM8, IM13, IM16, IM81, IM89, IM90, IM91 and IM106 complexes that have a relative concentration larger than 10−6 (1 ppm) with respect to IM21.

Mechanisms of the proton transfer reactions of 2-thioxanthine were investigated with both B3LYP/aug-cc-pVTZ//B3LYP/6-311+G** and PCM-B3LYP/aug-cc-pVTZ//B3LYP/6-311+G**. The models were designed with monohydrated and dihydrated microsolvent either with or without the presence of water solvent. The results show that the most feasible pathway is the reactions within the long range solvent surrounding via the N, O and S atoms in the pyrimidine ring with di-hydrated catalysis: N(7)H + 2H2O → IM90 → IM91 → P13 + 2H2O → IM92 → IM93 → P6 + 2H2O → IM72 → IM73 → P7 + 2H2O → IM109 → IM110 → P18 + 2H2O → IM113 → IM114 → P19 + 2H2O → IM115 → IM116 → P17 + 2H2O → IM107 → IM108 → N(9)H + 2H2O that has the highest barrier of 44.0 kJ mol−1 in the transition of IM90 to IM91 via TS54. The barrier is adequate for a reaction at room temperature that consists well with the experimental observations.


















Similar content being viewed by others
References
Ren HJ, Su KH, Liu Y, Wang X, Wang YL, Xiao J (2012) Proton transfer tautomerization mechanisms of 2-thioxanthine. Comput Theor Chem 982:40–46
Speina E, Ciela JM, Grziewicz MA, Laval J, Kazimierczuk Z, Tudek B (2005) Inhibition of DNA repair glycosylases by base analogs and tryptophan pyrolysate, Trp-P-1. Acta Biochim Pol 52:167–178
Elgemeie GH (2003) Thioguanine, mercaptopurine: their analogues and nucleosides as antimetabolites. Curr Pharm Des 9:2627–2642
Kouni MH (2003) Potential chemotherapeutic targets in the purine metabolism of parasites. Pharmacol Ther 99:283–309
Papakostas K, Georgopoulou E, Frillingos S (2008) Cysteine-scanning analysis of putative Helix XII in the YgfO xanthine permease. J Biol Chem 283:13666–13678
Mautner HG, Bergson G (1963) Some remarks on position-conditioned differences of the ultraviolet spectra and chemical reactivities of disubstituted pyrimidines and purines. Acta Chem Scand 17:1694–1704
Twanmoh LM, Wood HB, Driscoll JS (1973) NMR spectral characteristics of N-H protons in purine derivatives. J Heterocycl Chem 10:187–190
Civcir PU (2001) Tautomerism of 2-thioxanthine in the gas and aqueous phases using AM1 and PM3 methods. J Mol Struct (THEOCHEM) 546:163–173
Li BZ (2004) Density functional theory calculations on 2-thioxanthine. Acta Phys Chim Sin 20:1455–1458
Ahn DS, Lee S, Bongsoo K (2004) Solvent-mediated tautomerization of purine: single to quadruple proton transfer. Chem Phys Lett 390:384–388
Jeon IS, Ahn DS, Park SW, Lee S, Kim SK (2005) Structures and isomerization of serine in aqueous solution: computational study. Chem Phys Lett 403:72–76
Lee KM, Park SW, Jeon IS, Lee BR, Ahn DS, Lee SB (2005) Density functional theory study of acetonitrile-water clusters: structures and Infrared frequency shifts. Bull Korean Chem Soc 26:909–915
Park SW, Im S, Lee S, Desfrancois C (2007) Structure and stability of glycine-(H2O)3 cluster and anion: zwitterion vs. canonical glycine. Int J Quantum Chem 107:1316–1327
Yoon I, Seo K, Lee S, Lee Y, Kim B (2007) Conformational study of tyramine and its water clusters by laser spectroscopy. J Phys Chem A 111:1800–1807
Baymak MS, Vercoe KL, Zuman P (2005) Strong covalent hydration of terephthalaldehyde. J Phys Chem B 109:21928–21929
Ahn DS, Kang AR, Lee S, Kim B, Kim SK, Neuhauser D (2005) On the stability of glycine-water clusters with excess electron: implications for photoelectron. J Chem Phys 122:084310–084319
Gorb L, Leszczynski J (1998) Intramolecular proton transfer in mono- and dihydrated tautomers of guanine: an ab initio Post Hartree-Fock study. J Am Chem Soc 120:5024–5032
Kastas G (2012) Investigating the prototropic tautomerism in (E)-2-[(4-fluorophenyl)iminomethyl]- 5-methoxyphenol compound for solid state and solvent media by experimental and quantum computational tools. J Mol Struct 1017:38–44
Iglesias E (2003) Tautomerization of 2-acetylcyclohe-xanone.1.characterization of keto-enol/enolate equilibria and reaction rates in water. J Org Chem 68:2680–2688
Shen Z, Zhang YL, Jin FM, Zhou XF, Kishita A, Tohji K (2010) Hydrogen-transfer reduction of ketones into corresponding alcohols using formic acid as a hydrogen donor without a metal catalyst in high-temperature water. Ind Eng Chem Res 49:6255–6259
Riley BT, Long FA (1962) Long, deuterium isotope and solvent effects on the kinetics of the keto-enol interconversion of 2-acetylcyclohexanone. J Am Chem Soc 84:522–526
Ahn DS, Lee S, Kim B (2004) Solvent-mediated tautomerization of purine: single to quadruple proton transfer. Chem Phys Lett 390:384–388
Kim HS, Ahn DS, Chung SY, Kim SK, Lee S (2007) Tautomerization of adenine facilitated by water: computational study of microsolvation. J Phys Chem A 111:8007–8012
Okovytyy SI, Sviatenko LK, Gaponov AA, Kasyan LI, Tarabara IN, Leszczynski J (2010) DFT study on tautomerism of dihydro-2H-1,5-benzodiazepin-2-ones and dihydro-2H-1,5-benzodiazepine-2-thiones. Eur J Org Chem 2:280–291
Yuan XX, Wang YF, Wang X, Chen WB, Fossey JS, Wong NB (2010) An ab initio and AIM investigation into the hydration of 2-thioxanthine. Chem Cent J 4:1–19
Balta B, Aviyente V (2003) Solvent effects on Glycine. I. A supermolecule modeling of tautomerization via intramolecular proton transfer. J Comput Chem 24:1789–1802
Balta B, Aviyente V (2004) Solvent effects on Glycine II. Water-assisted tautomerization. J Comput Chem 25:690–703
Frisch MJ, Trucks GW, Schlegel HB, Scuseria G.E, Robb MA, Cheeseman JR, Scalmani G., Barone, V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd, JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG., Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowsk J, Fox DJ (2009) Gaussian 09, Revision A.02, Gaussian Inc., Wallingford, CT
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Chuang CH, Lien MH (2004) Ab initio study on the effects of the substituent and the functional group on the isomerization of H3CC(X)Y and H2C(X)CHY(Y = SiH2, PH, S; X = H, CH3, NH2, OH, F). J Phys Chem A 108:1790–1798
Li P, Bu Y (2004) Investigation of double proton transfer behavior between glycinamide and formamide using density functional theory. J Phys Chem A 108:10288–10295
Nagy PI, Tejada FR, Messer WS (2005) Theoretical studies of the tautomeric equilibria for five-member n-heterocyles in the gas phase and in solution. J Phys Chem B 109:22588–22602
Lamsabhi AM (2008) Specific hydration effects on oxo − thio triazepine derivatives. J Phys Chem A 112:1791–1797
Miertuš S, Scrocco E, Tomasi J (1981) Electrostatic interaction of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects. Chem Phys 55:117–129
Miertuš S, Tomasi J (1982) Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem Phys 65:239–245
Pascual-Ahuir JL, Silla E, Tuñón I (1994) GEPOL: an improved description of molecular-surfaces. 3. A new algorithm for the computation of a solvent-excluding surface. J Comput Chem 15:1127–1138
Cossi M, Barone V (2000) Solvent effect on vertical electronic transitions by the polarizable continuum model. J Chem Phys 112:2427–2435
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3093
Jeffrey P, Merrick DM, Leo R (2007) An evaluation of harmonic vibrational frequency scale factors. J Phys Chem A 111:11683–11700
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem 24:669–681
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
Acknowledgments
Part of the calculations were performed in the High Performance Computation Center of the Northwestern Polytechnical University. Support by the Basic Research Foundation of Northwestern Polytechnical University (Grant No. JC201269) and Wenli Special Foundation of Xi’an Science Technology Bureau (CXY1134WL13) is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 578 kb)
Rights and permissions
About this article
Cite this article
Ren, HJ., Su, KH., Liu, Y. et al. A theoretical investigation on the proton transfer tautomerization mechanisms of 2-thioxanthine within microsolvent and long range solvent. J Mol Model 19, 3279–3305 (2013). https://doi.org/10.1007/s00894-013-1858-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-013-1858-0