Abstract
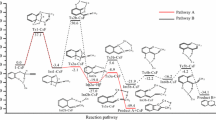
In the present research, the experimentally observed regioselectivity in Sonogashira synthesis of 6-(4-nitrobenzyl)-2-phenylthiazolo[3,2-b]1,2,4triazole has been modeled by means of density functional theory (DFT) employed to investigate the structural and thermochemical aspects of this synthesis in the gas and solution phases. Comparison of our calculated structural parameters of the title compound with the available X-ray crystallographical data demonstrate a reliable agreement. Then, the effect of two different solvents, DMF and ethanol, are examined via polarized continuum model calculations, showing a significant decrease in the computed values of the reaction enthalpy and free energy changes compared with the gas phase results. We have also considered two tautomeric structures of the intermediate species that it seems the mode of its intermolecular cyclization has an important role in regioselectivity of the final products. Moreover, all obtained results in the gas and solution phases also confirm that the synthesis of the title compound is thermodynamically more favorable than the other regioisomeric product. We also discuss the thermodynamical feasibility of this reaction at higher temperatures. Finally, we concentrate on the survey of substituent effect by choosing electron-withdrawing and electron-donating groups on the aryl iodide. Our calculated thermochemical data in the gas and solution phases indicate that the use of electron-withdrawing moieties is more favorable thermodynamically than electron-donating ones which has been previously concluded via the experimental elucidations.

A DFT study on regioselectivity






Similar content being viewed by others
References
Erol DD, Calis U, Demirdamar R, Yully N, Ertan M (1995) Synthesis and biological activities of some 3,6-disubstituted thiazolo[3,2-b][1,2,4]triazoles. J Pharm Sci 84:462–465
Odds FC, Abbott AB (1984) Antifungal relative inhibition factors: BAY 1–9139, bifonazole, butoconazole, isoconazole, itraconazole (R 51211), oxiconazole, Ro 14-4767/002, sulconazole, terconazole and vibunazole (BAY n-7133) compared in vitro with nine established antifungal agents. J Antimicrob Chemother 14:105–114
Doucet H, Hierso JC (2007) Palladium-based catalytic systems for the synthesis of conjugated enynes by sonogashira reactions and related alkynylations. Angew Chem 46:834–871
Chinchilla R, Najera C (2007) The Sonogashira reaction: a booming methodology in synthetic organic chemistry. Chem Rev 107:874–922
Dale DJ, Cartwright BA, Clark AJ, Mc Naib H (2001) Gas-phase pyrolysis of 4-amino-3-allylthio-1,2,4-triazoles: a new route to [1,3]thiazolo[3,2-b][1,2,4] triazoles. J Chem Soc Perkin Trans 1:424–428
Katritzky AR, Pastor A, Varonkov M, Steel PJ (2000) Novel one-step synthesis of Thiazolo[3,2-b]1,2,4-triazoles. Org Lett 2:429–431
Kochhar MM, Williams A (1972) Bicyclic triazoles. 1.3-(2-Furyl)-5-phenylthiazolo-2,3-c)-s-triazole. J Med Chem 15:332–333
Heravi MM, Kivanloo A, Rahimzadeh M, Bakavoli M, Ghassemzadeh M, Neumüller B (2005) Regioselective synthesis of 6-benzylthiazolo[3,2-b]1,2,4-triazoles during Sonogashira coupling. Tetrahedron Lett 46:1607–1610
Heck RF, Nolley JP Jr (1972) Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. J Org Chem 37:2320–2322
Milstein D, Stille JK (1978) A general, selective, and facile method for ketone synthesis from acid chlorides and organotin compounds catalyzed by palladium. J Am Chem Soc 100:3636–3638
Miyaura N, Suzuki A (1995) Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem Rev 95:2457–2483
Negishi E, Takahashi T, Babu S, Van Horn DE, Okukado N (1987) Palladium- or nickel-catalyzed reactions of alkenylmetals with unsaturated organic halides as a selective route to arylated alkenes and conjugated dienes: Scope, limitations, and mechanism. J Am Chem Soc 109:2393–2401
Sonogashira K, Tohda Y, Hagihara N (1975) A convenient synthesis of acetylenes: catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett 16:4467–4470
Sonogashira K (2002) Development of Pd-Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. J Organomet Chem 653:46–49
Hassan J, Sévignon M, Gozzi C, Schulz E, Lemaire M (2002) Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chem Rev 102:1359–1470
Negishi E, Anastasia L (2003) Palladium-catalyzed alkynylation. Chem Rev 103:1979–2017
Heravi MM, Sadjadi S (2009) Recent advances in the application of the Sonogashira method in the synthesis of heterocyclic compounds. Tetrahedron 65:7761–7775
Heravi MM, Tajbakhsh M, Ramezanian N (1998) Sulfuric acid adsorbed on silicagel: an inexpensive catalyst for regioselective cyclization of 3-propargylthio-s-triazole to 5-methylthiazolo[3,2-b]-s-triazole. Indian J Heterocycl Chem 7:309–310
Heravi MM, Tajbakhsh M (1998) Sodium hydroxide: a mild and inexpensive catalyst for the regioselective synthesis of 2-substituted 5-methylthiazolo[3,2-b]-s-triazoles. J. Chem. Res. 488–490
Heravi MM, Khademalfoghara HR, Sadeghi MM, Khaleghi Sh, Ghassemzadeh M (2006) Solvent free regioselective cyclization of 3-allylmercapto-1,2,4-triazoles to thiazolo[3,2-b]1,2,4-triazoles over sulfuric acid adsorbed on silica gel. 181:377–380
Becke AD (1993) A new mixing of Hartree-Fock and local density-functional theories. J Chem Phys 98:1372–1377
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B37:785–789
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A102:1995–2001
Amatore C, Carré E, Jutand A (1998) Evidence for an equilibrium between neutral and cationic arylpalladium(II) complexes in DMF. Mechanism of the reduction of cationic arylpalladium(II) complexes. Acta Chem Scand 52:100–106
Truhlar DG, Zhao Y (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functional. Theor Chem Account 120:215–241
Wadt WR, Hay PJ (1985) Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J Chem Phys 82:284–298
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Heravi MM, Kivanloo A, Rahimzadeh M, Bakavoli M, Ghassemzadeh M (2002) Heteroannulation through palladium catalysis: a novel cyclization leading to regioselective synthesis of 3-substituted thiazolo[3,2-C]1,2,4-triazin-5-ones. Phosphorus Sulfur Silicon 177:2491–2496
Heravi MM, Kivanloo A, Rahimzadeh M, Bakavoli M, Ghassemzadeh M, Neumüller B (2005) Regioselective synthesis of 3-benzylthiazolo[3,2-a]pyrimidones and 3-benzyl-thiazolo[3,2-c]pyrimidones through palladium-catalyzed heteroannulation of acetylenic compounds. Phosphorus Sulfur Silicon 180:2407–2417
Acknowledgments
The authors gratefully acknowledge the partial financial support received from the research council of Alzahra University. We have also thank to Prof. Mitra Ghassemzadeh for providing us X-ray crystallographical data. The first author is also grateful to Dr. M.H. Karimi-Jafari for his invaluable comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseinnejad, T., Heravi, M.M. & Firouzi, R. Regioselectivity in Sonogashira synthesis of 6-(4-nitrobenzyl)-2-phenylthiazolo[3,2-b]1,2,4-triazole: a quantum chemistry study. J Mol Model 19, 951–961 (2013). https://doi.org/10.1007/s00894-012-1639-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1639-1