Abstract
The extensive computation study was done to elucidate the mechanism of formation dibromoepoxide from cyclohexanone and bromoform. In this reaction, the formation of dihaloepoxide 2 is postulated as a key step that determines the distribution and stereochemistry of products. Two mechanistic paths of reaction were investigated: the addition of dibromocarbene to carbonyl group of ketone, and the addition of tribromomethyl carbanion to the same (C=O) group. The mechanisms for the addition reactions of dibromocarbenes and tribromomethyl carbanions with cyclohexanone have been investigated using ab initio HF/6-311++G** and MP2/6-311+G* level of theory. Solvent effects on these reactions have been explored by calculations which included a continuum polarizable conductor model (CPCM) for the solvent (H2O). The calculations showed that both mechanisms are possible and are exothermic, but have markedly different activation energies.

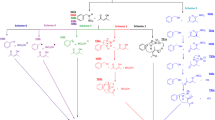
Mechanistic pathways for the reaction of cyclohexanone with bromoform and PE map for ionic mechanism (path 2).






Similar content being viewed by others
References
Barthel WF, Leon J, Hall SA (1954) J Org Chem 19:485–489
Leon J, Barthel WF, Hall SA (1954) J Org Chem 19:490–492
Reeve W, Fine LW (1964) J Org Chem 29:1148–1150
Palaty J, Abbott FS (1995) J Med Chem 38:3398–3406
Reeve W, Woods CW (1960) J Am Chem Soc 82:4062–4066
Reeve W (1971) Synthesis 3:131–138
Kuhl P, Mühlstädt M, Graefe J (1976) Synthesis 12:825–826
Merz A (1974) Synthesis 10:724–725
Corey EJ, Link JO (1992) J Am Chem Soc 114:1906–1908
Gill HS, Landgrebe JA (1983) J Org Chem 48:1051–1055
Peng L, Li QS, Fang WH, Fu CJ, Zhang J (2003) Chem Phys Lett 382:126–132
Vitnik VD, Ivanović MD, Vitnik ŽJ, Đorđević JB, Žižak ŽS, Juranić ZD, Juranić IO (2009) Synt Comm 39(8):1457–1471
Pliego JR Jr, De Almeida WB (1997) J Chem Phys 106:3582–3587
Vitnik ŽJ, Kiricojević VD, Ivanović MD, Juranić IO (2006) Int J Quant Chem 106(6):1323–1329
Hine J (1950) J Am Chem Soc 72:2438–2445
Hine J, Dowell AM Jr (1954) J Am Chem Soc 76:2688–2692
Curtiss LA, Raghavachari K, Pople JA (1993) J Chem Phys 98:1293–1298
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara AM, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision C.02. Gaussian Inc, Wallingford
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154–2161
Barone V, Cossi M (1998) J Phys Chem A 102:1995–2001
Acknowledgments
This work has been financially supported by Ministry of Education and Science, Republic of Serbia, under Grant No. 172035, and by High-Performance Computing Infrastructure for South East Europe’s Research Communities European project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vitnik, V.D., Vitnik, Ž.J. & Juranić, I.O. Carbenic vs. ionic mechanistic pathway in reaction of cyclohexanone with bromoform. J Mol Model 18, 4721–4728 (2012). https://doi.org/10.1007/s00894-012-1468-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1468-2