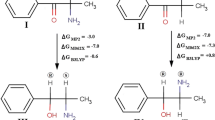
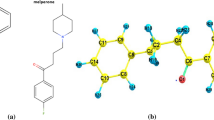
Abstract
A molecular modeling study on 16 1-benzyl tetrahydroisoquinolines (BTHIQs) acting as dopaminergic ligands was carried out. By combining molecular dynamics simulations with ab initio and density functional theory (DFT) calculations, a simple and generally applicable procedure to evaluate the binding energies of BTHIQs interacting with the human dopamine D2 receptor (D2 DR) is reported here, providing a clear picture of the binding interactions of BTHIQs from both structural and energetic viewpoints. Molecular aspects of the binding interactions between BTHIQs and the D2 DR are discussed in detail. A significant correlation between binding energies obtained from DFT calculations and experimental pKi values was obtained, predicting the potential dopaminergic effect of non-synthesized BTHIQs.











Similar content being viewed by others
References
Oloff S, Mailman RB, Tropsha A (2005) Application of validated QSAR models of D1 dopaminergic antagonist for database mining. J Med Chem 48:7322–7332
Sit SY, Xie K, Jacutin-Porte S, Boy KM, Seanz J, Taber MT, Gulwadi AG, Korpinen CD, Burris KD, Molski TF, Ryan E, Xu C, Verdoorn T, Johnson G, Nichols DE, Mailman RB (2004) Synthesis and SAR exploration of dinapsoline analogues. Bioorg Med Chem 12:715–734
Strange PG (1997) Dopamine receptors. Tocris Reviews 15. Tocris Cookson, Bristol
Zhang A, Neumeyer JL, Baldessarini RJ (2007) Recent progress in development of dopamine receptor subtype-selectiveagents: potential therapeutics for neurological and psychiatric disorders. Chem Rev 107:274–302
Protais P, Arbaoui J, Bakkali EH, Bermejo A, Cortes D (1995) Effects of various isoquinoline alkaloids on in vitro 3H-dopamine uptake. J Nat Prod 58:1475–1484
Bermejo A, Protais P, Blázquez MA, Rao KS, Zafra-Polo MC, Cortes D (1995) Dopaminergic isoquinoline alkaloids from rotos of Xylopia papuana. Nat Prod Res 6:57–62
Cabedo N, Protais P, Cassels BK, Cortes D (1998) Synthesis and dopamine receptor selectivity of the benzyltetrahydroisoquinoline, (R)-(+)-nor-roefractine. J Nat Prod 61:709–712
Cabedo N, Andreu I, Ramírez de Arellano MC, Chagraoui A, Serrano A, Bermejo A, Protais P, Cortes D (2001) Enantioselective syntheses of dopaminergic (R)- and (S)-benzyltetrahydroisoquinolines. J Med Chem 44:1794–1801
Berenguer I, El Aouad N, Andujar S, Romero V, Suvire F, Freret T, Bermejo A, Ivorra MD, Enriz RD, Boulouard M, Cabedo N, Cortes D (2009) Tetrahydroisoquinolines as dopaminergic ligands: 1-butyl-7-chloro-6-hydroxy-tetrahydroisoquinoline, a new compound with antidepressant-like activity in mice. Bioorg Med Chem 17:4968–4980
El Aouad N, Berenguer I, Romero V, Marín P, Serrano A, Andujar S, Suvire F, Bermejo A, Ivorra MD, Enriz RD, Cabedo N, Cortes D (2009) Structure–activity relationship of dopaminergic halogenated 1-benzyl-tetrahydroisoquinoline derivatives. Eur J Med Chem 44:4616–4621
Andreu I, Cabedo N, Torres G, Chagraoui A, Ramirez de Arellano MC, Gil S, Bermejo A, Valpuesta M, Portais P, Cortes D (2002) Syntheses of dopaminergic 1-cyclohexylmethyl-78-dioxygenated tetrahydroisoquinolines by selective heterogeneous tandem hydrogenation. Tetrahedron 58:10173–10179
Bermejo A, Andreu I, Suvire F, Léonce S, Caignard DH, Renard P, Pierré A, Enriz RD, Cortes D, Cabedo N (2002) Syntheses and antitumor targeting G1 phase of the cell cycle of benzoyldihydroisoquinoline. J Med Chem 45:5058–5068
Suvire FD, Andreu I, Bermejo A, Zamora MA, Cortes D, Enriz RD (2003) Conformational study of N-alkyl-benzyltetrahydroisoquinolines alkaloid. J Mol Struct THEOCHEM 666–667:109–116
Suvire FD, Cabedo N, Chagraoui A, Zamora MA, Cortes D, Enriz RD (2003) Molecular recognition and binding mechanism of N-alkyl-benzyltetrahydro-isoquinolines to the D1 dopamine receptor. A computational approach. J Mol Struc THEOCHEM 666–667:455–467
Andreu I, Cortes D, Protais P, Cassels BK, Chagraoui A, Cabedo N (2000) Preparation of dopaminergic N-Alkyl-benzyltetrahydroisoquinolines using a ‘One-Pot’ procedure in acid medium. Bioorg Med Chem 8:889–895
Doi S, Shirai N, Sato Y (1997) Abnormal products in the Bischler-Napieralski isoquinoline synthesis. J Chem Soc Perkin Trans 1:2217–2221
Andujar SA, Migliore de Angel BM, Charris JE, Israel A, Suárez-Roca H, López SE, Garrido MR, Cabrera EV, Visual G, Rosales C, Suvire FD, Enriz RD, Angel-Guío JE (2008) Synthesis dopaminergic profile and molecular dynamics calculations of N-aralkyl substituted 2-aminoindans. Bioorg Med Chem 16:3233–3244
Kalani MYS, Vaidehi N, Hall SE, Trabanino RJ, Freddolino PL, Kalani MA, Floriano WB, Wai Tak Kam V, Goddard WA III (2005) The predicted 3D structure of the human D2 dopamine receptor and the binding site and binding affinities for agonists and antagonists. Proc Natl Acad Sci USA 101:3815–3820
Becker OM, Marantz Y, Shacham S, Inbal B, Heifetz A, Kalid O, Bar-Haim S, Warshaviak D, Fichman M, Noiman SG (2004) Protein-coupled receptors: in silico drug discovery in 3D. Proc Natl Acad Sci USA 101:11304–11309
Micheli F, Bonanomi G, Blaney FE, Braggio S, Capelli AM, Checchia A, Curcuruto O, Damiani F, Di Fabio R, Donati D, Gentile G, Gribble A, Hamprecht D, Tedesco G, Terreni S, Tarsi L, Lightfoot A, Stemp G, MacDonald G, Smith A, Pecoraro M, Petrone M, Perini O, Piner J, Rossi T, Worby A, Pilla M, Valerio E, Griffante C, Mugnaini M, Wood M, Scott C, Andreoli M, Lacroix L, Schwarz A, Gozzi A, Bifone A, Ashby CR Jr, Hagan JJ, Heidbreder C (2007) 124-Triazol-3-yl-thiopropyl-tetrahydrobenzazepines: a series of potent and selective dopamine D3 receptor antagonists. J Med Chem 50:5076–5089
Ribeiro AA, Horta BAC, de Alencastro RB (2008) MKTOP: a program for automatic construction of molecular topologies. J Braz Chem Soc 19:1433–1435
Manzour A, Meng F, Meador-Woodruff JH, Taylor LP, Civelli O, Akil H (1992) Site-directed mutagenesis of the human dopamine D2 receptor. Eur J Pharm Mol Pharmacol Sec 227:205–214
Lan H, DuRand CJ, Teeter MM, Neve KA (2006) Structural determinants of pharmacological specificity between D1 and D2 dopamine receptors. Mol Pharmacol 69:185–194
Berendsen HJC, Van der Spoel D, Van Drunen R (1995) GROMACS: a message-passing parallel molecular dynamics implementations. Comput Phys Commun 91:43–56
Lindahl E, Hess B, van der Spoel D (2001) GROMACS 3.0: a package for molecular simulations and trajectory analysis. J Mol Model 7:306–317
van Buuren AR, Marrink SJ, Berendsen HJC (1993) A molecular dynamics study of the decane/water interface. J Phys Chem 36:9206–9212
Mark AE, van Helden SP, Smith PE, Janssen LHM, van Gunsteren WF (1994) Convergence properties of free energy calculations. A-cyclodextrin complexes as a case study. J Am Chem Soc 116:6293–6302
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
van Buuren AR, Berendsen HJC (1993) Molecular dynamics simulation of the stability of a 22 residue alpha-helix in water and 30% trifluoroethanol. Biopolymers 33:1159–1166
Liu H, Muller-Plathe F, van Gunsteren WF (1995) A force field for liquid dimethyl sulfoxide and physical properties of liquid dimethyl sulfoxide calculated using molecular dynamics simulation. J Am Chem Soc 117:4363–4366
Miyamoto S, Kollman PA (1992) SETTLE—an analytical version of the SHAKE and RATTLE algorithm for rigid water models. J Comput Chem 13:952–962
Berendsen HJC, Postma HJC, Van Gunsteren WF, Hermans WF (1981) Interaction models for water in relation to protein hydration. In: Pullman B (ed) Intermolecular forces. Reidel, Dordrecht, pp 331–342
Darden T, York D, Pedersen L (1993) Particle mesh Ewald—an N.log(n) method for Ewald sums in large systems. J Chem Phys 98:10089–10092
Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG (1995) A smooth particle mesh Ewald method. J Chem Phys 103:8577–8593
Luty B, Tironi IG, van Gunsteren WF (1995) Lattice–sum methods for calculating electrostatic interactions in molecular simulations. J Chem Phys 103:3014–3021
Zimmerman K (1991) All purpose molecular mechanics simulator and energy minimizer. J Comput Chem 12:310–319
Ferguson DM (1995) Parameterization and evaluation of a flexible water model. J Comput Chem 16:501–511
Berendsen HJC, Postma JPM, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690
Hess B, Bekker H, Berendsen HJC, Fraaije JG (1997) E.M. LINCS: a linear constraint solver for molecular simulations. J Comput Chem 18:1463–1472
Kampmann T, Mueller DS, Mark AE, Young PR, Kobe B (2006) The role of histidine residues in low-pH-mediated viral membrane fusion. Structure 14:1481–1487
Teeter MM, Froimowitz MF, Stec B, Durand CJ (1994) Homology modeling of the dopamine D2 receptor and its testing by docking of agonists and tricyclic antagonists. J Med Chem 37:2874–2888
Neve KA, Cumbay MG, Thompson KR, Yang R, Buck DC, Watts VJ, Durand CJ, Teeter MM (2001) Modeling and mutational analysis of a putative sodium-binding pocket on the dopamine D2 receptor. Mol Pharmacol 60:373–381
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, Revision B.05. Gaussian Inc, Pittsburgh
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Hjerde E, Dahl SG, Sylte I (2005) Atypical and typical antipsychotic drug interactionswith the dopamine D2 receptor. Eur J Med Chem 40:185–194
Cho W, Taylor LP, Mansour A, Akil A (1995) Hydrophobic residues of the D2 dopamine receptor are important for binding and signal transduction. J Neurochem 65:2105–2115
Cox BA, Henningsen RA, Spanoyannis A, Neve RL, Neve KA (1992) Contributions of conserved serine residues to the interactions of ligands with dopamine D2 receptors. J Neurochem 59:627–635
Wiens BL, Nelson CS, Neve KA (1998) Contribution of serine residues to constitutive and agonist-induced signaling via the D2Sdopamine receptor: evidence for multiple agonist-specific active conformations. Mol Pharmacol 54:435–444
Wilcox RE, Huang WH, Brusniak MYK, Wilcox DM, Pearlman RS, Teeter MM, Durand CJ, Wiens BL, Neve KA (2000) CoMFA-based prediction of agonist affinities at recombinant wild type versus serine to alanine point mutated D2 dopamine receptors. J Med Chem 43:3005–3019
Page CS, Bates PA (2008) Can MM-PBSA calculations Predict the specifiicties of protein kinase inhibitors? J Comput Chem 27:1990–2007
Mertz KM (2010) Limits of free energy computation for protein–ligand interactions. J Chem Theor Comput 6:1769–1776
Breneman CM, Wiberg KB (1990) Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J Comput Chem 11:361–373
Politzer P, Murray JS, Clark T (2010) Halogen bonding: an electrostatically-driven highly directional noncovalent interaction. Phys Chem Chem Phys 12:7748–7757
Bader RFW (1990) Atoms in molecules. A quantum theory. Oxford University Press, Oxford
Popelier PLA (1999) Atoms in molecules. An introduction. Pearson, Harlow
Acknowledgments
Grants from Universidad Nacional de San Luis (UNSL) partially supported this work. This research was also supported by the Spanish “Ministerio de Educación y Ciencia” grant SAF 2007–63142. S.A.A. thanks a postdoctoral fellowship of CONICET-Argentina. R.D.E. is a member of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET-Argentina) staff.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andujar, S., Suvire, F., Berenguer, I. et al. Tetrahydroisoquinolines acting as dopaminergic ligands. A molecular modeling study using MD simulations and QM calculations. J Mol Model 18, 419–431 (2012). https://doi.org/10.1007/s00894-011-1061-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-011-1061-0