Abstract
Objective
To conduct a systematic review of the published scientific evidence to evaluate the efficacy of nonsurgical periodontal therapy (NSPT) in treating periodontitis in patients with concurrent systemic conditions (diabetes, CVD, erectile dysfunction, chronic kidney disease, rheumatoid arthritis, polycystic ovarian syndrome, obesity, pregnancy). We hypothesised that NSPT results in better periodontal outcomes when compared to untreated controls after follow-up.
Materials and methods
A systematic search (PUBMED/EMBASE) was conducted from 1995 to 2023 to identify randomised controlled trials (RCTs) with a minimum follow-up of 3 months. The primary outcome was the difference in mean probing depth (PD), and the secondary outcomes were mean clinical attachment loss (CAL), percentage of sites with PD ≤ 3 mm (%PD ≤ 3 mm) and percentage of sites with bleeding on probing (%BOP) between the treated and untreated control group in patients with comorbidities.
Results
The electronic search resulted in 2,403 hits. After removing duplicates, 1,565 titles and abstracts were screened according to the eligibility criteria, resulting in 126 articles for full-text screening. Following this, 44 studies were analysed. Restricting to studies with low bias or some concerns, NSPT group demonstrated a 0.55 mm lower mean PD (95%CI: −0.69; −0.41) after 3 months compared to the control group.
Conclusion
Compared to the untreated controls, NSPT notably reduced mean PD, mean CAL, and %BOP while increasing %PD ≤ 3 mm in patients with concurrent systemic conditions. These findings suggest that NSPT is also an effective procedure in managing periodontitis in patients with concurrent systemic conditions.
Trial registration
This systematic review was registered under the protocol registration number CRD42021241517/PROSPERO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first and second steps in periodontitis treatment are performed non-surgically (NSPT) combined with oral hygiene instructions [1]. Typically, this results in the reduction of probing depth, gain in clinical attachment, resolution of inflammation and arrests the progression of periodontitis [2,3,4].
Although NSPT is the gold standard for periodontal treatment [5], there is a paucity of randomized clinical trials (RCTs), specifically designed to assess the efficacy of NSPT versus no treatment in individuals with comorbidities [2, 6]. Several authors have argued that a wealth of literature has demonstrated efficacy in other contexts, and a negative conclusion would be unfair [2]. In the last decade, health policy stakeholders have asked for reliable, evidence-based data to recommend or include NSPT in insurance or reimbursement schemes. The Canadian Agency for Drugs and Technologies in Health [7] concluded that NSPT improved periodontal outcomes in adult patients with varying severity of periodontitis with or without systemic diseases but not in patients with incipient periodontitis within a three-month observation period. Based on a thorough literature review, the German Institute for Quality and Efficiency in Healthcare deemed NSPT as a procedure with an uncertain benefit [8].
Since the 1990s, multiple epidemiological, experimental, and interventional studies have revealed that periodontitis may impact systemic health [9]. Through the emergence of “periodontal medicine”, the periodontal community has become involved in the medical field and aimed to demonstrate the impact of periodontal treatment on other chronic inflammatory medical conditions [10]. To substantiate their hypotheses, the periodontal community had to perform state-of-the-art medical experiments with an untreated, periodontally diseased control arm. To overcome the ethical dilemma, the periodontal treatment of the control arm was delayed, until the medical question, e.g., change in biomarker levels, was expected to be answered. Because of a presumptive progression of periodontitis due to non-treatment, the periodontal community restricted the length of delayed treatment to only 3 months in most cases. Few RCTs were performed longer than 6 months [11, 12]. Although these studies were designed to answer different research questions, they can be used to answer the question of NSPT’s efficacy.
This introduction highlights that health policy around the globe asks whether NSPT improves periodontal parameters better than no treatment. Due to the emergence of periodontal medicine, many RCTs that compared immediate and delayed NSPT are available to address the question: "Does NSPT enhance periodontal parameters in patients with periodontitis compared to delayed treatment in individuals with concurrent systemic conditions?”. Even if many RCTs are available, the limitation is that the information stems from patients with concurrent systemic conditions and not healthy individuals. This study aimed to perform a meta-analysis of relevant “periodontal medicine” literature to evaluate the efficacy of NSPT compared to no treatment, supragingival scaling (SGS) or oral hygiene instruction (OHI) (control group) in periodontitis patients with concurrent systemic conditions.
Methods
Standards of reporting
The protocol of this systematic review is in accordance with the “Preferred Reporting Items for Meta-Analysis (PRISMA) statement” [13] and is intended to address the following question: "Does NSPT enhance periodontal parameters in patients with periodontitis in comparison to no treatment (no Tx), supragingival scaling (SGS), or oral hygiene instructions (OHI) in individuals with concurrent systemic disease or condition (comorbidity)?" (Protocol registration: CRD42021241517/PROSPERO) [14].
Eligibility criteria
Studies fulfilling the following criteria were eligible for inclusion: 1) RCTs involving human subjects from 18 years onward, suffering from periodontitis associated with a systemic disease or condition; 2) studies which used NSPT as monotherapy without local or systemic antibiotics, without other physical adjunctive interventions or without periodontal surgery; 3) studies with no treatment, OHI or SGS as control; 4) studies reporting mean PD with a minimum of 3 months post-treatment follow-up. Articles published in languages other than English were excluded.
Source of information and search strategy
Keywords and MeSH terms were selected, and electronic search strategies were developed for PubMed and Embase (Appendix Table 1). A literature search was also conducted using keywords on Google Scholar. Additionally, a manual search of the references from the included studies was performed. The publications were collected and organised using a reference manager (EndNote X7, Thomson Reuters) and duplicates were excluded. Two reviewers (PJ and VP) independently searched studies published between 01.01.1995 and 30.09.2023.
Selection process
The study selection process was done independently by two reviewers (PJ and VP) in two phases. Phase-1: two reviewers screened the titles and abstracts of all identified reports, based on the inclusion/exclusion criteria. Phase-2: the full texts of the selected studies were evaluated according to the eligibility criteria. In case of disagreements, a consensus was reached by consulting the third reviewer (TK). Excel spreadsheets were used to record the decisions (Appendix Table 2).
Data collection process and data items
Two reviewers (PJ and PP) independently extracted relevant data from the included studies, such as study population, interventions, comparisons, reported outcomes, baseline and follow-up values and conclusions. This information was filled in Excel spreadsheets to provide an overview of the available data. Discrepancies were solved by consensus discussion with VP and TK, and the values were updated.
The primary outcome was mean PD. Mean clinical attachment loss (CAL) in mm, percentage of sites with bleeding on probing (%BOP) and percentage of sites with PD ≤ 3 mm (%PD ≤ 3 mm) were the secondary outcomes assessed in this review. For all outcomes, means and standard deviations were extracted at 3 and 6 months for NSPT and control groups (Appendix Table 3).
Risk of bias assessment
Two reviewers (PJ, VP) independently assessed risk of bias for included RCTs, according to the Cochrane Collaboration risk-of-bias tool for randomized trials (RoB2) [15]. Each study was graded according to five domains: randomization (D1), deviation (D2), missing data (D3), outcome measurement (D4) and selective reporting (D5), and an overall score for risk of bias was assigned. Discrepancies raised were discussed with two researchers (TK and BH) until an agreement was reached.
Effect measures and synthesis methods
The mean difference and the 95% confidence interval (95% C.I.) for all outcomes between the test and the control arm at the 3- and/or 6-month follow-ups were calculated. Negative estimates favour the NSPT group over the control group except for %PD ≤ 3 mm. Studies with a low risk of bias or rated as some concerns were grouped together and compared to studies with high risk of bias when computing pooled estimates or when plotting forest plots. When median values were reported, they were converted into mean values, and the missing standard deviations were imputed using the average standard deviation from the available studies as prescribed by The Cochrane Collaboration [16].
We performed subgroup analyses in patients with comorbidities, such as, type 2 diabetes, cardiovascular diseases (CVD), erectile dysfunction, pregnancy, and rheumatoid arthritis (RA). Systemic diseases/conditions (obesity, polycystic ovarian syndrome (PCOS), chronic obstructive pulmonary disease (COPD), and chronic kidney disease (CKD)) with less than two studies were excluded from subgroup analyses. To examine the efficacy of NSPT, we calculated the change in means between the pre- and post-treatment values within the NSPT group for the abovementioned variables. Heterogeneity was quantified using the I2 statistic, and the publication bias was tested using Egger’s test (Egger et al., 1997) and illustrated outcomes through funnel plots. Random-effects meta-regression was performed by modelling the pre- and post-treatment values of all outcomes on the type of comorbidity, risk of bias, and year of publication.
Results
Study selection
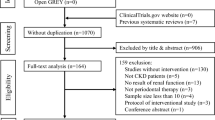
The initial search yielded 2,403 articles. After removing duplicates, 1,565 records were screened by title and abstract and 126 full-text articles were assessed for eligibility. A total of 44 studies met the inclusion criteria (Appendix Fig. 1). These studies were published between 1995 and 2023.
Study characteristics
Methodology
All but four trials were single-centre trials. Of the included trials, 25 had a 3-month follow-up period, 20 included a 6-month follow-up, two had a 12-month follow-up, and one had a 24-month follow-up. The 44 trials were performed in different countries as follows: Australia (1), Austria (1), Brazil (8), Chile (1), China (8), Egypt (1), Greece (1), India (7), Iran (2), Japan (1), Jordan (1), Malaysia (2), Pakistan (1), Spain (1), Turkey (2), the United Kingdom (2), the USA (3), and Vietnam (2).
Participant characteristics
Overall, 44 studies (3382 patients, ages ranging from 22 to 68 years) were included in the meta-analysis. All patients had periodontitis, although the severity varied. Of the included trials, 21 were conducted in patients with diabetes, 9 in patients with CVD, 6 in pregnant women, 2 in men with ED, 1 in patients with CKD, 2 in patients with RA, 1 in patients with COPD 1 in women with PCOS, and 1 in obese patients.
Periodontal interventions and measures
In 26 RCTs, NSPT was performed with curettes and/or ultrasonic instruments, and 19 RCTs did not report the instruments used. NSPT was performed in one to five sessions; however, 15 trials did not report the number of sessions. In the NSPT group, five studies used a chlorhexidine mouth rinse as an adjunct. In the control arm, 21 studies reported no treatment, nine used SGS, and 15 used OHI. In 37 RCTs, periodontal outcomes were measured at four or six sites per tooth. Further information about teeth examined, probe used, and study conclusions are summarized in Table 1.
Twenty-seven studies involving 2,530 participants and thirteen studies involving 1,292 patients reported mean CAL at the 3-month and 6-month examinations, respectively. Thirty studies involving 2,826 patients and sixteen studies involving 1,470 patients reported mean PD at the 3-month and 6-month examinations, respectively. Twenty-three studies involving 2,333 patients and fifteen studies involving 1,424 patients reported %BOP at the 3-month and 6-month examinations, respectively.
Risk of bias
The risk of bias assessment was summarized based on the intention-to-treat or per-protocol principle (Fig. 1). The risk of bias for all domains was low in 17 trials. In the remaining 19 trials, the risk of bias was of some concern because it was not explicitly described whether sequence generation and/or allocation concealment was adequately done. Nine studies had a high risk of bias, because protection against performance and detection biases was inadequate, as personnel and outcome assessment were unblinded or not mentioned. Blinding patients to the intervention was impossible due to the nature of the interventions. Evaluations of a potential publication bias revealed a significant small-study effect for PD reduction. Twenty-six studies analysed all patients, 19 studies only post-treatment data from patients who were available at a follow-up visit, and 21 studies reported an analysis based on intention-to-treat. Compliance with treatment was not a concern given that most studies performed SRP once at baseline. Because the selected studies were not planned to evaluate the effect of NSPT versus no NSPT, we did not include this aspect in our bias assessment.
Meta-analysis
Probing depth
In total, 2,826 patients from 30 studies with 3 months data with a high risk of bias, some concerns or low risk were analysed. Restricting 23 studies to those with low or some bias concerns showed a significant mean difference in mean PD of −0.55 mm (95% C.I.: −0.69; −0.41) favouring NSPT (Fig. 2). Including all studies, irrespective of bias, did not change this mean difference. A subgroup analysis with diabetic patients yielded similar results, with a mean PD difference of −0.49 mm (95% C.I.: −0.68; −0.31) in favour of NSPT. Restricting studies to those with CVD patients and low or some concerns of bias (2 studies with 151 CVD patients) yielded a mean PD difference of −0.86 mm (95% C.I.: −1.06; −0.66) in favour of NSPT (Table 2).
At 6 months, using data from 15 trials (1,424 patients) with low or some concerns of bias, a mean PD difference of −0.49 mm (95% C.I.: −0.68; −0.30) was observed between the NSPT and the control group (Fig. 2). In diabetic patients (8 trials with low bias or some concerns and 1155 patients) a mean PD difference of −0.47 mm (95% C.I.: −0.65; −0.29) was observed (Table 2). We judged the overall level of certainty in the evidence to be moderate based on the evidence profile. Irrespective of performed analyses, heterogeneity varied between 97% at 3 months and 93% at 6 months including studies with high bias.
Clinical attachment level
In total, 2241 patients from 22 studies with 3-month data with low bias or some concerns were analysed. A statistically significant mean CAL difference of −0.51 mm (95% C.I.: −0.65; −0.37) in favour of NSPT was observed. Including five studies (289 patients) with high bias did not materially change the mean CAL difference (−0.30 mm (95% C.I.: −0.70; 0.09) (Fig. 3). Subgroup analysis yielded similar results: 14 studies with 1347 diabetic patients with low bias or some concern, the mean CAL difference was −0.54 mm (95% C.I.: −0.72; −0.36) in favour of NSPT. From two CVD (151 patients) studies with low bias or some concern, the mean CAL difference was −0.56 mm (95% C.I.: −0.97; −0.15) (Table 2).
At 6 months, 12 trials (1244 patients) with low bias or some concern showed a mean CAL difference of -0.49 mm (95% C.I.: −0.71; −0.26) in favour of NSPT (Fig. 3). Eight trials involving 1058 diabetic patients yielded a mean CAL difference of −0.49 mm (95% C.I.: −0.68; −0.30) in favour of NSPT (Table 2). Including all RCTs, the study heterogeneity was 90% at 3 months and 83% at 6 months.
Bleeding on probing
In total, 19 studies (2,134 patients) with 3-month data with low bias or some concerns were analysed. %BOP was significantly lower (−23.94% (95% C.I.: −30.35%; −17.53%)) in NSPT compared to the control group. Including studies with high bias did not change the results (−23.90% (95% C.I.: −29.27; −18.53)) (Fig. 4). The subgroup analysis of nine diabetes studies (1138 patients) yielded similar results: the mean %BOP difference was −18.70% (95% C.I.: −26.87; −10.53), whereas in three CVD studies (190 patients), the mean %BOP difference was -35.00% (95% C.I.: −47.47; −22.53) (Table 2).
At 6 months, in 15 trials with low bias or some concerns, including 1,422 patients, a mean %BOP difference of −27.22% (95% C.I.: −34.66; −19.78) was observed in favour of NSPT (Fig. 4). 10 trials with 1120 diabetic patients yielded a mean %BOP difference of −26.44% (95% C.I.: −33.75; −19.13) in favour of NSPT (Table 2). Study heterogeneity was 98% at 3 months and 93% at 6 months, including all RCTs, showing considerable significance.
Percentage of sites with probing depth ≤ 3 mm
Because of the limited number of studies at 6-month follow-up with this information, the results in this section were limited to the 3-month follow-up. Eight studies (one with high risk of bias) provided means and standard deviations. The overall mean difference in %PD ≤ 3 mm between NSPT and the control group was 13.73% (95% C.I.: 5.20; 22.26). When unreported standard deviations were imputed, 16 studies (3 with high risk of bias) were included. The mean difference in %PD ≤ 3 mm was 14.98% (95% C.I.: 8.48; 21.48) in studies with low/some concern bias and 14.36% (95% C.I.: 8.83; 19.89) in all a. However, the pooled estimates were lower (10.98% (95% C.I.: 1.62; 20.35)) in high-risk studies (Fig. 5).
Forest plot showing the mean differences of the percentage of sites with probing depths ≤ 3 mm (%PD ≤ 3 mm) sorted according to risk of bias assessment (low/some concerns vs. high) at 3 months; a including the studies where missing standard deviations were imputed; b only studies with complete information
Pre-and post-treatment results
Irrespective of bias and treatment arm, mean PD at baseline varied between 1.21 ± 0.27 mm and 6.59 ± 1.50 mm (Appendix Table 3). NSPT reduced mean PD with a mean pre-post difference of 0.56 mm (95% C.I.: 0.46; 0.66) and 0.58 mm (95% C.I.: 0.40; 0.76) at 3 and 6 months, respectively (Appendix Fig. 2). Mean CAL reduced with a mean pre-post difference of 0.50 mm (95% C.I.: 0.38; 0.62) and 0.41 mm (95% C.I.: 0.21; 0.60) at 3 and 6 months, respectively. %BOP reduced with a mean pre-post difference of 29.92 (95% C.I.: 23.97; 35.87) and 32.28 (95% C.I.: 26.04; 38.52) at 3 and 6 months, respectively. In the NSPT group, %PD ≤ 3 mm increased by 17.32% (95% C.I.;23.80; 10.84) between baseline and the 6-month examination.
Meta-regression
We performed random-effects meta-regression analyses including all studies with 3- or 6-month follow-up data (Appendix Table 4 and 5, respectively). None of the factors were significantly associated with the meanPD at 3-month follow-up; but only a comorbidity type (PCOS: β = −0.743 (95% C.I.: −1.411; −0.076)) was found to be significantly associated with mean PD at 6-month follow-up (Appendix Table 4 and Appendix Table 5).
Discussion
This systematic review aimed to summarise the current literature on the efficacy of NSPT compared to no or minimal periodontal treatment in patients with comorbidities. Consistent with previous reviews on this topic, our primary outcome was mean PD, while our secondary outcomes included mean CAL, percentage BOP and percentage of sites with PD ≤ 3 mm assessed at 3 and 6 months. We acknowledge that mean PD may not have clear clinical relevance, but most included studies reported mean PD across all sites. In contrast, only very few reported the percentage of sites with PD 4–5 mm or ≥ 6 mm, which more accurately depicts the clinical situation. NSPT showed a 0.55 mm (95% C.I.: −0.69, −0.41) lower mean PD at the 3-month examination than the control group when studies were restricted to those with low bias or some concerns. The difference in mean PD attenuated to −0.49 mm (95% C.I.: −0.68, −0.30) at the 6-month examination.
Even when studies with a high risk of bias were included in the analysis, there was no change in mean PD difference. Regardless of the underlying comorbidity (diabetes, CVD, pregnancy), mean PD differences were consistent. Analyses of secondary outcomes (mean CAL, %BOP and %PD ≤ 3 mm) support our conclusion that NSPT reduced periodontal inflammation in periodontitis patients with comorbidities. The significant variation of baseline mean PD levels reflect the different inclusion criteria for periodontitis (Appendix Table 3). Although the RCTs included in our meta-analysis were primarily not designed to answer the above formulated question, namely the effect of NSPT in comparison to no treatment, SGS or OHI on periodontal conditions, we feel confident that our conclusion is robust. From our perspective, these results provide a definitive ‘yes’ that NSPT is an effective therapeutic measure in terms of clinical outcomes.
Overall, few previous reviews have investigated the question of whether NSPT is superior to no treatment, SGS or OHI, but results did not provide definitive conclusions due to the ethical implications of withholding periodontal therapy in the control group. A first review that tried to shed light on this question found two studies in which the treatment arm gained 0.22 mm more mean CAL compared to untreated controls [2, 61, 62]. In addition, this review included an observational study with two arms: 79 periodontally diseased subjects with no periodontal treatment and 108 patients treated with NSPT were monitored for one year. Compared to baseline, mean PD reduced by 0.50 ± 0.04 mm and mean CAL decreased by 0.44 ± 0.05 mm in the treatment group; in the untreated group mean PD decreased by 0.04 ± 0.05 mm, while mean CAL decreased by 0.21 ± 0.21 mm [63]. These data are in line with our results.
An open question remains as to whether the statistically significant difference in mean PD and mean CAL of 0.5 mm between the test and control group at the final examination or the pre-post mean differences of about 0.57 and 0.53 mm for mean PD and mean CAL in the test group, respectively, are clinically relevant. Extent values give a better understanding of the clinical reality: the pre-post %PD ≤ 3 mm in the test group increased by about 18.04%, from 66.21% to 83.63%, whereas it was materially zero in the control group. In the treatment arm pre-post %BOP was reduced from 53.2% to 23.1%, whereas in the control arm, it only decreased from 51.7% to 46.9%. Although the patients included in this meta-analysis were less severely diseased than those in a multicentre RCT with 200 patients (Harks et al. 2015), the resolution of inflammation exhibited comparable healing trends. The NSPT arm of this multicentre RCT showed a decrease in mean PD from 3.5 ± 0.8 mm to 2.7 ± 0.7 mm, %BOP was reduced from 34.2 ± 18.1% to 19.6 ± 14.9%, and %PD ≤ 3 mm increased from 59.2 ± 18.1% to 79.1 ± 15.9% (Harks et al. 2015). These data align very well with the data reported here. From our perspective, these values reflect clinically notable results, but they are still far from meeting the criteria for a successfully treated periodontitis patient as defined by the 2017 Workshop [64], should only exhibit BOP in < 10% of sites and have no sites with PD ≥ 4 mm and bleeding on probing. These studies suggest that even under institutional conditions, it may be difficult to achieve such a threshold.
In 2019, the European Federation of Periodontology commissioned a meta-analysis on the efficacy of NSPT [6]. The authors restricted their inclusion criteria to patients without comorbidities and found only one study, which did not allow for any robust conclusions to be drawn. To still answer this basic question, they analysed studies with different treatment protocols and reported the pre-post-treatment change in mean PD. Their reported results are based on a mixture of all measured sites with only moderate pockets, which prevents comparing their results with ours.
To answer whether NSPT outcomes achieved in medically compromised patients are inferior to those achieved in systemically healthy patients, we compared our results with data extracted from 53 reports with 1,474 systemically healthy periodontitis patients who underwent NSPT [65]. Three months after NSPT, the initial mean PD of 3.9 mm was reduced by 0.78 mm (95% C.I: 0.76–0.79) and the mean CAL gain was 0.65 mm (95% C.I: 0.63–0.67).
To graphically support the comparability of the short-term results in treating patients with and without comorbidity, baseline values of mean PD and CAL were associated with the corresponding values 3 months after NSPT. The slope of the association of mean PD or mean CAL did not differ between systemically healthy individuals and those with a comorbidity (Fig. 6). Although this comparison does not allow for any statistical inference, our results suggest that NSPT in medically compromised patients may produce similar results as in systemically healthy patients.
Irrespective of the comorbidity status, baseline values of mean PD and mean CAL were strongly associated with the corresponding measures at 3-months after NSPT. Study groups with higher mean PD at baseline exhibited a higher mean PD after therapy, whereas higher values of mean PD at baseline were concomitantly associated with greater reductions in mean PD over 3 months. Regarding the shape of the association, no significant differences between systemically healthy and diabetes groups were observed. In analyses of mean CAL, analogous results were obtained. The circle sizes represent the respective study sizes. For detailed information about the studies included in the arm with healthy subjects, please refer to Kocher et al. 2018. [66]
One strength of this review, which contributes to the robustness of the conclusion, is the high external validity base, as the patients participating in the RCTs were representative of the general population because they were not recruited in a dental school but rather from hospitals with different specialties.
This meta-analysis has several limitations. First, these studies were performed in periodontal institutions with presumably high technical scaling skills. Therefore, the present review describes the efficacy of the intervention rather than its effectiveness and does not reflect periodontal treatment in the community. Second, this meta-analysis provides robust estimates for NSPT efficacy only for a 3- and 6-month period, which is definitely too short to determine if NSPT has a long-term effect. One single-centre study and one large multicentre study reported stable mean PD reduction or mean CAL gain after NSPT without antibiotics after 12 or 27.5 months [67, 68]. On the other hand, treatment effects after 3 months are not blurred by periodontal maintenance measures. Third, with the delayed treatment design, the control arm was often offered supragingival cleaning and OHIs to motivate the patients to stay in the RCT instead of no treatment. But even this very first treatment step of OHI might cause a considerable resolution of periodontitis [69]. Thus, only considering the difference between periodontal variables between the test and control arm at the final visit may be misleading because it neglects the influence of improved supragingival plaque control either to the professional motivation and instruction and/or the removal of supragingival calculus. Fourth, included studies were designed for research questions other than the one we set for this review. According to the bias assessment, most studies had some concerns or a high risk of bias. However, the D1 and D4 domains were of utmost importance for this review, and both these domains had little bias. Fifth, only limited information about the number of sessions or time spent on NSPT or OHI was provided. Sixth, information about drug intake was sparse and too diverse to consider its impact on treatment outcomes. However, because all the studies included in this review are RCTs, the impact of medications should be the same in the control and treatment arms. Seventh, information on smoking was not available in 21 studies. Thus, we could not address the confounding effects of smoking on treatment outcomes.
Conclusion
There was a clinically relevant decrease in mean PD, mean CAL, and %BOP while having an increase in %PD ≤ 3 mm. Therefore, despite some limitations, this review’s findings suggest that NSPT is an effective procedure for managing periodontitis in patients with systemic diseases, which might be comparable with systemically healthy patients.
Data Availability
No new data was generated for this review. All the data used for the meta-analysis could be obtained directly from the respective publications. As a result, data sharing does not apply in this case.
References
Sanz M et al (2020) Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J Clin Periodontol 47(Suppl 22):4–60. https://doi.org/10.1111/jcpe.13290
Van der Weijden GA, Timmerman MF (2002) A systematic review on the clinical efficacy of subgingival debridement in the treatment of chronic periodontitis. J Clin Periodontol 29(Suppl 3):55–71. https://doi.org/10.1034/j.1600-051x.29.s3.3.x. (discussion 90-1)
Sanz I et al (2012) Nonsurgical treatment of periodontitis. J Evid Based Dent Pract 12(3 Suppl):76–86. https://doi.org/10.1016/s1532-3382(12)70019-2
Smiley CJ et al (2015) Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc 146(7):508–24.e5. https://doi.org/10.1016/j.adaj.2015.01.028
Cobb CM, Sottosanti JS (2021) A re-evaluation of scaling and root planing. J Periodontol 92(10):1370–1378. https://doi.org/10.1002/jper.20-0839
Suvan J et al (2020) Subgingival instrumentation for treatment of periodontitis. A systematic review. J Clin Periodontol 47(Suppl 22):155–175. https://doi.org/10.1111/jcpe.13245
Canadian Agency for Drugs and Technologies in Health (2016) Dental scaling and root planing for periodontal health: a review of the clinical effectiveness, Cost-effectiveness, and Guidelines, in CADTH Rapid Response Reports. Canadian Agency for Drugs and Technologies in Health, Ottawa
IQWIG (2018) (Bewertung der systematischen Behandlung von Parodontopathien) Evaluation of the systematic treatment of periodontal diseases. [Article in German], Cologne. https://www.iqwig.de/projekte/n15-01.html
Genco RJ, Sanz M (2020) Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontol 2000 83(1):7–13. https://doi.org/10.1111/prd.12344
Beck JD et al (2019) Periodontal medicine: 100 years of progress. J Dent Res 98(10):1053–1062. https://doi.org/10.1177/0022034519846113
Zhou X et al (2014) Effects of periodontal treatment on lung function and exacerbation frequency in patients with chronic obstructive pulmonary disease and chronic periodontitis: a 2-year pilot randomized controlled trial. J Clin Periodontol 41(6):564–572. https://doi.org/10.1111/jcpe.12247
D'Aiuto F et al (2018) Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol 6(12):954–965. https://doi.org/10.1016/s2213-8587(18)30038-x
Moher D et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
Schiavo JH (2019) PROSPERO: an international register of systematic review protocols. Med Ref Serv Q 38(2):171–180. https://doi.org/10.1080/02763869.2019.1588072
Sterne JAC et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Higgins J, Deeks J (2011) Chapter 6: Choosing effect measures and computing estimates of effect. In: Cochrane Handbook for Systematic Reviews of Interventions, Cochrane. https://training.cochrane.org/handbook/current/chapter-06
Rodrigues Amorim Adegboye A et al (2021) Exploratory efficacy of calcium-vitamin D milk fortification and periodontal therapy on maternal oral health and metabolic and inflammatory profile. Nutrients 13(3):783. https://doi.org/10.3390/nu13030783
Caneiro-Queija L et al (2019) Non-surgical treatment of periodontal disease in a pregnant caucasian women population: adverse pregnancy outcomes of a randomized clinical trial. Int J Environ Res Public Health 16(19):3638. https://doi.org/10.3390/ijerph16193638
Das AC et al (2019) Adjunctive effect of doxycycline with conventional periodontal therapy on glycemic level for chronic periodontitis with type 2 diabetes mellitus subjects. J Contemp Dent Pract 20(12):1417–1423
El-Makaky Y, AbdallaHawwam S, Hifnawy T (2020) Salivary tumor necrosis factor-alpha to detect the severity of erectile dysfunction: a randomized clinical trial. Oral Dis 26(7):1548–1557. https://doi.org/10.1111/odi.13380
Eltas A et al (2013) The effect of periodontal treatment in improving erectile dysfunction: a randomized controlled trial. J Clin Periodontol 40(2):148–154. https://doi.org/10.1111/jcpe.12039
Fiorini T et al (2013) Effect of nonsurgical periodontal therapy on serum and gingival crevicular fluid cytokine levels during pregnancy and postpartum. J Periodontal Res 48(1):126–133. https://doi.org/10.1111/j.1600-0765.2012.01513.x
Ide M et al (2003) Effect of treatment of chronic periodontitis on levels of serum markers of acute-phase inflammatory and vascular responses. J Clin Periodontol 30(4):334–340. https://doi.org/10.1034/j.1600-051x.2003.00282.x
Kamil W et al (2011) Effects of nonsurgical periodontal therapy on C-reactive protein and serum lipids in Jordanian adults with advanced periodontitis. J Periodontal Res 46(5):616–621. https://doi.org/10.1111/j.1600-0765.2011.01380.x
Kaur PK et al (2015) Periodontal and glycemic effects of nonsurgical periodontal therapy in patients with type 2 diabetes stratified by baseline HbA1c. J Oral Sci 57(3):201–211. https://doi.org/10.2334/josnusd.57.201
Kiran M et al (2005) The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol 32(3):266–272. https://doi.org/10.1111/j.1600-051X.2005.00658.x
Kolte RA et al (2023) Effect of nonsurgical periodontal therapy on metabolic control and systemic inflammatory markers in patients of type 2 diabetes mellitus with stage III periodontitis. Contemp Clin Dent 14(1):45–51. https://doi.org/10.4103/ccd.ccd_514_21
Koromantzos PA et al (2011) A randomized, controlled trial on the effect of non-surgical periodontal therapy in patients with type 2 diabetes. Part I: effect on periodontal status and glycaemic control. J Clin Periodontol 38(2):142–7. https://doi.org/10.1111/j.1600-051X.2010.01652.x
Masi S et al (2018) Mitochondrial oxidative stress, endothelial function and metabolic control in patients with type II diabetes and periodontitis: A randomised controlled clinical trial. Int J Cardiol 271:263–268. https://doi.org/10.1016/j.ijcard.2018.05.019
Milanesi FC et al (2023) Effect of periodontal treatment on glycated haemoglobin and metabolic syndrome parameters: a randomized clinical trial. J Clin Periodontol 50(1):11–21. https://doi.org/10.1111/jcpe.13717
Moeintaghavi A et al (2012) Non-surgical periodontal therapy affects metabolic control in diabetics: a randomized controlled clinical trial. Aust Dent J 57(1):31–37. https://doi.org/10.1111/j.1834-7819.2011.01652.x
Pinho M et al (2009) Relationship between periodontitis and rheumatoid arthritis and the effect of non-surgical periodontal treatment. Braz Dent J 20(5):355–364. https://doi.org/10.1590/s0103-64402009000500001
Ribeiro J, Leão A, Novaes AB (2005) Periodontal infection as a possible severity factor for rheumatoid arthritis. J Clin Periodontol 32(4):412–416. https://doi.org/10.1111/j.1600-051X.2005.00689.x
Sadatmansouri S, Sedighpoor N, Aghaloo M (2006) Effects of periodontal treatment phase I on birth term and birth weight. J Indian Soc Pedod Prev Dent 24(1):23–26. https://doi.org/10.4103/0970-4388.22831
Seinost G et al (2020) Periodontal treatment and vascular inflammation in patients with advanced peripheral arterial disease: a randomized controlled trial. Atherosclerosis 313:60–69. https://doi.org/10.1016/j.atherosclerosis.2020.09.019
Singh S et al (2008) The effect of periodontal therapy on the improvement of glycemic control in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Int J Diabetes Dev Ctries 28(2):38–44. https://doi.org/10.4103/0973-3930.43097
Telgi RL et al (2013) Efficacy of nonsurgical periodontal therapy on glycaemic control in type II diabetic patients: a randomized controlled clinical trial. J Periodontal Implant Sci 43(4):177–182. https://doi.org/10.5051/jpis.2013.43.4.177
Tran TT et al (2021) Effect of two nonsurgical periodontal treatment modalities in type 2 diabetes mellitus patients with chronic periodontitis: a randomized clinical trial. J Contemp Dent Pract 22(11):1275–1280
Vidal F et al (2009) Periodontal therapy reduces plasma levels of interleukin-6, C-reactive protein, and fibrinogen in patients with severe periodontitis and refractory arterial hypertension. J Periodontol 80(5):786–791. https://doi.org/10.1902/jop.2009.080471
Wang Y et al (2020) A randomized controlled trial of the effects of non-surgical periodontal therapy on cardiac function assessed by echocardiography in type 2 diabetic patients. J Clin Periodontol 47(6):726–736. https://doi.org/10.1111/jcpe.13291
Zhou SY et al (2013) Effect of non-surgical periodontal therapy on serum levels of TNF-a, IL-6 and C-reactive protein in periodontitis subjects with stable coronary heart disease. Chin J Dent Res 16(2):145–151
Akram Z et al (2017) Effect of nonsurgical periodontal treatment on clinical periodontal variables and salivary resistin levels in obese Asians. J Oral Sci 59(1):93–102. https://doi.org/10.2334/josnusd.16-0127
Chen L et al (2012) Effects of non-surgical periodontal treatment on clinical response, serum inflammatory parameters, and metabolic control in patients with type 2 diabetes: a randomized study. J Periodontol 83(4):435–443. https://doi.org/10.1902/jop.2011.110327
Deepti et al (2017) Effect of non-surgical periodontal therapy along with myo-inositol on high-sensitivity C-reactive protein and insulin resistance in women with polycystic ovary syndrome and chronic periodontitis: a randomized controlled trial. J Periodontol 88(10):999–1011. https://doi.org/10.1902/jop.2017.170121
Engebretson SP et al (2013) The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. JAMA 310(23):2523–2532. https://doi.org/10.1001/jama.2013.282431
Fang F et al (2015) The clinical response and systemic effects of non-surgical periodontal therapy in end-stage renal disease patients: a 6-month randomized controlled clinical trial. J Clin Periodontol 42(6):537–546. https://doi.org/10.1111/jcpe.12411
Hada DS et al (2015) Effect of non-surgical periodontal treatment on clinical and biochemical risk markers of cardiovascular disease: a randomized trial. J Periodontol 86(11):1201–1211. https://doi.org/10.1902/jop.2015.150249
Kapellas K et al (2014) Effect of periodontal therapy on arterial structure and function among aboriginal australians: a randomized, controlled trial. Hypertension 64(4):702–708. https://doi.org/10.1161/hypertensionaha.114.03359
López NJ, Smith PC, Gutierrez J (2002) Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: a randomized controlled trial. J Periodontol 73(8):911–924. https://doi.org/10.1902/jop.2002.73.8.911
Mizuno H et al (2017) The effects of non-surgical periodontal treatment on glycemic control, oxidative stress balance and quality of life in patients with type 2 diabetes: A randomized clinical trial. PLoS ONE 12(11):e0188171. https://doi.org/10.1371/journal.pone.0188171
Montenegro MM et al (2019) Randomized controlled trial of the effect of periodontal treatment on cardiovascular risk biomarkers in patients with stable coronary artery disease: Preliminary findings of 3 months. J Clin Periodontol 46(3):321–331. https://doi.org/10.1111/jcpe.13085
Offenbacher S et al (2006) Effects of periodontal therapy during pregnancy on periodontal status, biologic parameters, and pregnancy outcomes: a pilot study. J Periodontol 77(12):2011–2024. https://doi.org/10.1902/jop.2006.060047
Offenbacher S et al (2009) Results from the Periodontitis and Vascular Events (PAVE) Study: a pilot multicentered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease. J Periodontol 80(2):190–201. https://doi.org/10.1902/jop.2009.080007
Pham TAV et al (2022) Nonsurgical periodontal treatment improved the type 2 diabetes mellitus status in smokers: a randomized controlled trial. Diabetes Res Clin Pract 194:110150. https://doi.org/10.1016/j.diabres.2022.110150
Qureshi A et al (2021) Clinical efficacy of scaling and root planing with and without metronidazole on glycemic control: three-arm randomized controlled trial. BMC Oral Health 21(1):253. https://doi.org/10.1186/s12903-021-01620-1
Raman RP et al (2014) Effect of nonsurgical periodontal therapy verses oral hygiene instructions on type 2 diabetes subjects with chronic periodontitis: a randomised clinical trial. BMC Oral Health 14:79. https://doi.org/10.1186/1472-6831-14-79
Saffi MAL et al (2018) Periodontal therapy and endothelial function in coronary artery disease: a randomized controlled trial. Oral Dis 24(7):1349–1357. https://doi.org/10.1111/odi.12909
Wang S et al (2017) Glycemic control and adipokines after periodontal therapy in patients with Type 2 diabetes and chronic periodontitis. Braz Oral Res 31:e90. https://doi.org/10.1590/1807-3107BOR-2017.vol31.0090
Wang Y et al (2017) Periodontal treatment modulates gene expression of endothelial progenitor cells in diabetic patients. J Clin Periodontol 44(12):1253–1263. https://doi.org/10.1111/jcpe.12806
Wu Y et al (2015) Effect of non-surgical periodontal treatment on visfatin concentrations in serum and gingival crevicular fluid of patients with chronic periodontitis and type 2 diabetes mellitus. J Periodontol 86(6):795–800. https://doi.org/10.1902/jop.2015.140476
Burgett FG et al (1992) A randomized trial of occlusal adjustment in the treatment of periodontitis patients. J Clin Periodontol 19(6):381–387. https://doi.org/10.1111/j.1600-051x.1992.tb00666.x
Chapple IL et al (1995) Effect of instrument power setting during ultrasonic scaling upon treatment outcome. J Periodontol 66(9):756–760. https://doi.org/10.1902/jop.1995.66.9.756
Machtei EE et al (2000) Outcome variables in periodontal research: means and threshold-based site changes. J Periodontol 71(4):555–561. https://doi.org/10.1902/jop.2000.71.4.555
Dietrich T et al (2019) Periodontal diagnosis in the context of the 2017 classification system of periodontal diseases and conditions – implementation in clinical practice. Br Dent J 226(1):16–22. https://doi.org/10.1038/sj.bdj.2019.3
Kocher T et al (2018) Periodontal complications of hyperglycemia/diabetes mellitus: Epidemiologic complexity and clinical challenge. Periodontol 2000 78(1):59–97. https://doi.org/10.1111/prd.12235
Kocher T et al (2018) Periodontal complications of hyperglycemia/diabetes mellitus: Epidemiologic complexity and clinical challenge. Periodontology 2000 78(1):59–97. https://doi.org/10.1111/prd.12235
Borges I et al (2017) Different antibiotic protocols in the treatment of severe chronic periodontitis: a 1-year randomized trial. J Clin Periodontol 44(8):822–832. https://doi.org/10.1111/jcpe.12721
Harks I et al (2015) Is progression of periodontitis relevantly influenced by systemic antibiotics? A clinical randomized trial. J Clin Periodontol 42(9):832–842. https://doi.org/10.1111/jcpe.12441
Preus HR, Al-Lami Q, Baelum V (2020) Oral hygiene revisited. The clinical effect of a prolonged oral hygiene phase prior to periodontal therapy in periodontitis patients. A randomized clinical study. J Clin Periodontol 47(1):36–42. https://doi.org/10.1111/jcpe.13207
Funding
Open Access funding enabled and organized by Projekt DEAL. Supported by the institution.
Author information
Authors and Affiliations
Contributions
TK and VP substantially contributed to the conception or design of the work. JP, PP, VP, CP and TK contributed to the acquisition; JP, VP and BH contributed to the analysis and interpretation of data. JP, VP and TK drafted the work. BH and JS revised the work critically for important intellectual content. All authors approved the final version of the manuscript and are accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethical approval
Not necessary.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thomas Kocher and Vinay Pitchika contributed equally as last authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joseph, P., Prabhakar, P., Holtfreter, B. et al. Systematic review and meta-analysis of randomized controlled trials evaluating the efficacy of non-surgical periodontal treatment in patients with concurrent systemic conditions. Clin Oral Invest 28, 21 (2024). https://doi.org/10.1007/s00784-023-05392-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-023-05392-6