Abstract
Objectives
To investigate the intraosseous arterial pathways and anastomoses in the alveolar aspects of the maxilla in order to better understand the arterial scattering pattern.
Materials and methods
Eleven cadavers were selected for macroscopic intraosseous arterial analyses by corrosion casting. The red-colored acrylic resin was injected into the external carotid arteries. The specimens were kept in an enzymatic solution at 36 °C for about 60 days, depending on the process progression. After removal of the soft tissues and drying, the bone was macerated by potassium hydroxide to analyze the course and the mean diameters of the intraosseous anastomoses.
Results
Vertico-oblique and horizontal intraosseous arteries and anastomoses between the greater palatine-, posterior superior alveolar-, and infraorbital arteries were detected. The vertico-oblique anastomoses were found on the anterolateral wall of the maxilla and the alveolar crest with a mean diameter of 0.46 mm; nevertheless, the horizontal (transalveolar) anastomoses were identified in the interdental septum/alveolar crest with the mean diameter of 0.41 mm. From the horizontal anastomoses, small intraseptal branches supplied the territory of the alveolar socket in various directions.
Conclusions
The localization of intraosseous arterial anastomoses is critical in implant-related surgeries, predominantly to maintain proper circulation.
Clinical relevance
Based on vertico-oblique and transalveolar anastomoses, simultaneous buccal- and palatal flap elevation (particularly on the palatal side) should be avoided to minimize patient morbidity and intra- or postoperative complications. Moreover, preserving transverse loops in the interdental septum is essential during implant surgeries, which can significantly influence collateral periosteal and osteal circulation to prevent ischemia.
Similar content being viewed by others
Introduction
Oral rehabilitation with dental implants is nowadays a well-established treatment modality for replacing missing teeth to restore chewing function and esthetics [1]. Despite the relatively high predictability of implant therapy and high costs, patient perceptions of success and patient-reported outcome measures have become increasingly significant in implant dentistry.
In perioimplant-related surgeries, the morphological and vascularization factors in the maxilla convey an intricate pattern that can result in challenging circumstances [2,3,4]. Through a better understanding of the topography of the mucosal and periosteal branches of critical arteries, such as the posterior superior alveolar artery (PSAA), greater palatine artery (GPA), and infraorbital artery (IOA) with their anastomoses [5,6,7], clinicians can avoid or minimize complications related to intense intra- and postoperative hemorrhage, compromised wound healing, and flap necrosis. Previous investigations regarding subepithelial connective tissue grafting revealed that flap design is significantly affected by the morphology of the palatal vault and the topography of the arterial pathway of GPA with critical anastomoses [7,8,9,10].
Regarding the intraosseous vascularization patterns in the bony alveolus and implant area, the literature indicates various endoscopic investigations assisting in comprehending vascular canals [11, 12]. Particularly, Engelke et al. [11] demonstrated in vivo patterns of vascular canals (50 microns) and hemorrhage by utilizing microscopic bone analysis and support immersion alveoloscopy [11]. Furthermore, via morphometric investigation, they detected deficient vascular canals and unmineralized bone in the implant zones corresponding to the extraction sockets [11]. During or prior to implant placement, ridge augmentation is frequently inevitable. According to Tan et al. [13], after tooth loss, the vertical dimension of the bone can decrease by 11–22% (0.84 ± 0.62 mm mesially and 0.80 ± 0.71 mm distally) after six months, and the horizontal dimension by 29–63% (3.79 ± 0.23 mm), respectively [13]. Based on the fact of alveolar resorption, the allocation pattern of the intraosseous and periosteal arteries might be altered. Horizontal ridge augmentation is typical in the esthetic zone and premolar area, sometimes along with sinus floor elevation in the posterior region [14]. However, vertical ridge augmentation is predominantly performed in the esthetic zone [15, 16]. In the maxilla, donor sites for autogenous bone harvesting mainly include the maxillary tuberosity and the anterior nasal spine, which contain dense arterial supply and can be impaired during harvesting and alveolar reconstruction. Furthermore, hemorrhage can occur during autogenous bone harvesting from the maxillary tuberosity or during implant placement, especially in the vicinity of the incisive canal, following damage to the nasopalatine artery (NPA) [17].
Several studies have investigated the macroscopic vascularization of the PSAA, IOA, and GPA [5,6,7,8,9]. Regardless, the explicit branching and anastomoses pattern of intraosseous and periosteal arteries remains demanding.
Thus, the present study aimed to analyze the intraosseous vascularization and anastomoses of the arterial network in the alveolar aspects of the anterolateral wall of the maxilla and hard palate, as well as the alveolar crest (interdental septum) by the corrosion casting technique to provide indications for improving the safety of implant-related surgery.
Materials and methods
Eleven maxillae (7 males, 4 females; 50–85 years of age) were selected for the macroscopic intraosseous arterial investigation (2 dentate, 2 partially edentulous in premolar and molar area, and 7 edentulous). Fresh bodies were provided for corrosion casting analysis. The specimens were selected based on the proper conditions of the vessels without known vascular-related comorbidities. The bodies were donated for science and research to the Department of Anatomy, Histology and Embryology, Semmelweis University, Budapest, Hungary, according to Hungarian approval laws of anatomical donation (approval number: 110/2020.(VII.07.)). In the first step, the external carotid arteries (ECAs) were irrigated by saline solution. Then, acrylic resin (ACRIFIX 190 (2 R 0190), Evonik Industries AG., Germany) was mixed with red Akemi Akepox coloring paste (AKEMI GmbH., Nurnberg, Germany). The mixture was introduced into the ECAs. Subsequently, the head specimens were kept at room temperature for 24 h for complete resin polymerization. In order to dissolve the soft tissues around the injected vessels, the specimens were kept in the enzymatic solution (Somat gold 12 actions (Henkel AG., Germany)) at 36 °C for about 60 days, depending on the process progression. Every 15–20 days, the solution was replaced with great care to avoid a fracture of the intermediate corrosion cast. After all soft tissues were dissolved, the specimens were carefully washed and left in cold water for 3 days to eliminate the remaining chemicals. After drying, the bony areas were macerated by 2–4% KOH to analyze the intraosseous branches. The diameters of vertico-oblique- and horizontal intraosseous arterial anastomoses were measured at the level of the alveolar crest/interdental septum by Topex caliper (31C616 Vernier Caliper 200 mm, Warsaw, Poland), and the mean diameters of anastomoses were compared.
Results
In all investigated specimens, the following arterial connections were discovered: vertico-oblique intraosseous anastomoses (vertico-oblique loop) between branches of PSAA/IOA with the GPA were discovered following maceration of the anterolateral wall of the maxilla and the alveolar crest (Fig. 1). In the interdental septum/alveolar crest, horizontal bony perforating anastomoses (transverse loop) between the GPA branches and the PSAA/IOA branches were identified (Fig. 2). The vertico-oblique anastomoses presented as more prominent in diameter than the horizontal anastomoses (Table 1). Furthermore, the size of the horizontal intraosseous perforating arteries was thicker on the palatal side than the buccal arteries, suggesting that the direction of the blood flow might be from the palatal side toward the buccal/labial side (Fig. 2). From the transverse loop, small intraseptal branches created a network at the territory of the alveolar socket and in a coronal direction they were detected in the alveolar crest (Fig. 2).
Vertico-oblique intraosseous anastomoses (vertico-oblique loop). (a) Anterolateral view of the maxilla, vertico-oblique intraosseous branches of the infraorbital artery (IOA), and the greater palatine artery (GPA), concealed in the anterolateral surface of the maxilla. (b) Anterolateral view of the maxilla with a magnified view. After removing the hard tissue through the cautious maceration, vertico-oblique loop between IOA and GPA in the alveolar crest and socket initiated the arterial network. (c) Inferior view from the side of the hard palate; the anastomoses are marked with arrows
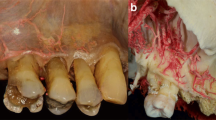
Horizontal intraosseous anastomoses (transverse loop). (a) Overview of left front teeth after extraction, transalveolar anastomoses in the interdental septum marked with arrows. (b) Marked transverse loop; the intraosseous artery at the palatal aspect exhibits a larger dimension than the vestibular side, and small intraseptal branches are observed
Discussion
Collateral vascularization should be preserved during implant-related surgeries to maintain proper circulation [18, 19]. Inadequate blood supply or damaged bilateral blood flow in the surgical area might increase the risk of ischemia and necrosis in soft and hard tissues [20].
In this study, the corrosion casting method permitted to observe that (i) vertico-oblique intraosseous branches of PSAA/IOA supplying the alveolar aspects of the anterolateral wall of the maxilla formed anastomoses with the intraosseous branches of GPA (vertico-oblique loop); (ii) horizontal perforating transalveolar anastomoses between the branches of GPA with PSAA/IOA (transverse loop) in the alveolar crest/interdental septum presented small intraseptal branches. These intraseptal branches supplied the territory of the alveolar socket and moved toward the alveolar crest, periosteum, and the gingival papilla. Based on our investigations, intraseptal branches assembled anastomoses with the supraperiosteal arteries and the vessels of the periodontal ligament. Furthermore, this network might impact supplying the circulation of the gingiva and can substantially influence the outcomes and complication rate of implant-related surgeries in the maxilla. A previous study from our group regarding maxillary vestibule arterial supply emphasized the bony penetrating branches in connection with transverse mucoperiosteal anastomoses on the buccal aspect in the premolar/molar region which were perfusing the buccal wall and interalveolar septum [21]. The current investigation mainly demonstrates the course of perforating intraosseous arteries and analyzes anastomoses in the alveolar aspects of the maxilla. Conceivably the intraosseous branches may connect with the transverse periosteomucosal network; so in implant-related surgeries, if these anastomoses are mostly preserved, they can play a critical role in the proper perfusion around implant territory. Furthermore, the intraosseous arterial pattern of the NPA branches in the alveolar crest and hard palate should be investigated individually, which could be critical for circulation in the esthetic zone in implant- and maxillofacial related surgeries.
The mean arterial diameter of vertico-oblique anastomoses was slightly more prominent than horizontal anastomoses. However, several physiological factors, such as bone resorption and vascular pressure changes, might influence the dimensions of the anastomoses. Due to the limited numbers of the maxillae, detailed statistical analyses were not assembled. Based on our determinations, horizontal perforating arteries drive from the palate toward the buccal cortex and create an arterial network around the alveolar socket. Preserving transverse loop vascularization in the interdental septum is essential during immediate implant surgeries. The findings also suggest that palatal flap elevation should be avoided or minimized in order to decrease patient morbidity and complications.
During ridge augmentation, the interruption of palatal transalveolar arteries should be avoided in order to facilitate the optimal incorporation of the bone grafts/bone substitutes. Similarly, it seems to be desirable that the implant dimension should be less than the alveolar socket from the mesiodistal and buccopalatal aspects, to minimize the pressure on the bony walls and transalveolar anastomoses thus preventing insufficient circulation and resorption of bony tissue. Additionally, the distance between implants should be at least 2–3 mm to have sufficient interimplant vascularization, especially in the esthetic zone [22, 23]. Nevertheless, small palatal perforating vessels have a poor prognosis and might rupture after excessive flap elevation and mobilization.
Ultimately, in sinus floor elevation, especially in the lateral window technique, it is inevitable to elevate a buccal mucoperiosteal flap and the Schneiderian membrane [24]. Consequently, the blood flow of the alveolar process is bidirectionally compromised due to the interruption of vertico-oblique branches by soft/hard tissue manipulation. The present analysis indicates vertico-oblique loops and transverse loops between GPA and PSAA/IOA are critical anastomoses during lateral sinus floor elevation. Damaging these intraosseous anastomoses might result in disturbed graft blood supply and osteogenesis. The graft vascularization can be ensured by elevating the Schneiderian membrane and reaching the mesial wall; four intact bony walls can provide excellent blood supply utilizing the arterial loops.
Conclusion
A thorough knowledge of the intraosseous arterial supply is essential to prevent intra- and postoperative complications in implant-related surgeries. Our study highlighted that the intraosseous vascularization of the alveolar aspects of the maxilla strongly relies on the vertico-oblique and transalveolar intraosseous spongious branches of GPA and PSAA/IOA, forming the vertico-oblique and the transverse loop anastomoses. Therefore, simultaneous buccal- and palatal flap elevation should be avoided to minimize patient morbidity and reduce complications during maxillary osseous surgeries.
References
Duong HY, Roccuzzo A, Stähli A, Salvi GE, Lang NP, Sculean A (2022) Oral health-related quality of life of patients rehabilitated with fixed and removable implant-supported dental prostheses. Periodontol 2000 88:201–237
Ramanauskaite A, Becker J, Sader R, Schwarz F (2019) Anatomic factors as contributing risk factors in implant therapy. Periodontol 2000 81:64–75
Sanz-Sánchez I, Sanz-Martín I, Ortiz-Vigón A, Molina A, Sanz M (2022) Complications in bone-grafting procedures: classification and management. Periodontol 2000 88:86–102
Zucchelli G, Wang HL, Chambrone L (2022) Complications and treatment errors in periodontal and implant therapy. Periodontol 2000. https://doi.org/10.1111/prd.12442
Rosano G, Taschieri S, Gaudy JF, Weinstein T, Del Fabbro M (2010) Maxillary sinus vascular anatomy and its relation to sinus lift surgery. Clin Oral Implants Res 22:711–715
Kqiku L, Biblekaj R, Weiglein AH (2016) Location of the extraosseous and intraosseous arterial anastomosis of the maxillary sinus in edentulous specimens. Clin Oral Investig 20:2311–2314
Reiser GM, Bruno JF, Mahan PE, Larkin LH (1996) The subepithelial connective tissue graft palatal donor site: anatomic considerations for surgeons. Int J Periodontics Restorative Dent 16:130–137
Fu JH, Hasso DG, Yeh CY, Leong DJ, Chan HL, Wang HL (2011) The accuracy of identifying the greater palatine neurovascular bundle: a cadaver study. J Periodontol 82:1000–1006
Shahbazi A, Grimm A, Feigl G, Gerber G, Székely AD, Molnár B, Windisch P (2019) Analysis of blood supply in the hard palate and maxillary tuberosity—clinical implications for flap design and soft tissue graft harvesting (a human cadaver study). Clin Oral Investig 23:1153–1160
Tavelli L, Barootchi S, Stefanini M, Zucchelli G, Giannobile WV, Wang HL (2022) Wound healing dynamics, morbidity, and complications of palatal soft-tissue harvesting. Periodontol 2000 00:1–30
Engelke W, Lazzarini M, Stühmer W, Beltrán V (2015) Support immersion endoscopy in post-extraction alveolar bone chambers: a new window for microscopic bone imaging in vivo. PLoS One 10:e0145767
Arraño D, Chaparro A, Lazzarini M, Acuña-mardones P, Engelke W, Beltrán V (2020) In situ endoscopic analysis of bone microstructure and vascularization in post-extraction sites immediately after a minimally invasive vertical tooth extraction in teeth with different periodontal status: a preliminary study. Int J Morphol 38:1735–1741
Tan WL, Wong TL, Wong MC, Lang NP (2012) A Systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implants Res 23(Suppl 5):1–21
Lundgren S, Cricchio G, Hallman M, Jungner M, Rasmusson L, Sennerby L (2017) Sinus floor elevation procedures to enable implant placement and integration: techniques, biological aspects and clinical outcomes. Periodontol 2000 73:103–120
Pelo S, Boniello R, Gasparini G, Longobardi G, Amoroso PF (2007) Horizontal and vertical ridge augmentation for implant placement in the aesthetic zone. Int J Oral Maxillofac Surg 36:944–948
Urban IA, Monje A, Lozada JL, Wang HL (2017) Long-term evaluation of peri-implant bone level after reconstruction of severely atrophic edentulous maxilla via vertical and horizontal guided bone regeneration in combination with sinus augmentation: a case series with 1 to 15 years of loading. Clin Implant Dent Relat Res 19:46–55
Jacobs R, Quirynen M, Bornstein MM (2014) Neurovascular disturbances after implant surgery. Periodontol 2000 66:188–202
Mikecs B, Vág J, Gerber G, Molnár B, Feigl G, Shahbazi A (2021) Revisiting the vascularity of the keratinized gingiva in the maxillary esthetic zone. BMC Oral Health 21(1):160
Fazekas R, Molnár E, Lohinai Z, Dinya E, Tóth Z, Windisch P, Vág J (2018) Functional characterization of collaterals in the human gingiva by laser speckle contrast imaging. Microcirculation 25:e12446
Lim G, Lin GH, Monje A, Chan HL, Wang HL (2018) Wound healing complications following guided bone regeneration for ridge augmentation: a systematic review and meta-analysis. Int J Oral Maxillofac Implants 33:41–50
Shahbazi A, Feigl G, Sculean A, Grimm A, Palkovics D, Molnár B, Windisch P (2021) Vascular survey of the maxillary vestibule and gingiva-clinical impact on incision and flap design in periodontal and implant surgeries. Clin Oral Investig 25:539–546
Traini T, Novaes AB, Piattelli A, Papalexiou V, Muglia VA (2010) The relationship between interimplant distances and vascularization of the interimplant bone. Clin Oral Implants Res 21:822–829
Buser D, Martin W, Belser UC (2004) Optimizing esthetics for implant restorations in the anterior maxilla: anatomic and surgical considerations. Int J Oral Maxillofac Implants 19(Suppl):43–61
Shao Q, Li J, Pu R, Feng Y, Jiang Z, Yang G (2021) Risk factors for sinus membrane perforation during lateral window maxillary sinus floor elevation surgery: a retrospective study. Clin Implant Dent Relat Res 23:812–820
Acknowledgements
The authors would like to thank the Department of Anatomy, Histology, and Embryology, Semmelweis University, for supporting the investigation. The authors are also grateful to Dr. Lorenzo Tavelli, Department of Oral Medicine, Immunity, and Infection, Division of Periodontology, Harvard School of Dental Medicine, Boston, USA, for critically reviewing the manuscript. All authors have viewed and agreed to the submission.
Funding
Open access funding provided by Semmelweis University.
Author information
Authors and Affiliations
Contributions
Arvin Shahbazi created the research and wrote the main manuscript text. Arvin Shahbazi, Gábor Baksa, and Sebastian Gschwindt prepared specimens and figures. Sándor Bogdán analyzed data and participated in preparing the manuscript. Anton Sculean, Bálint Molnár, and János Vág inspected the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The bodies were donated for science and research to the Department of Anatomy, Histology, and Embryology, Semmelweis University, Budapest, Hungary, according to Hungarian approval laws of anatomical donation (approval number: 110/2020.(VII.07.)). No additional ethical approval was required for this study.
Informed consent
No patient consent was required for this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shahbazi, A., Sculean, A., Baksa, G. et al. Intraosseous arterial alteration of maxilla influencing implant-related surgeries. Clin Oral Invest 27, 5217–5221 (2023). https://doi.org/10.1007/s00784-023-05141-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05141-9