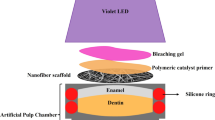
Abstract
Objective
The study aims to assess the effects of a 10% H2O2 bleaching gel with different MnO2 concentrations on the bleaching efficacy (BE), degradation kinetics (DK) of H2O2, and trans-amelodentinal cytotoxicity (TC).
Materials and methods
Standardized bovine enamel/dentin disks (n = 96) were placed in artificial pulp chambers, and the bleaching gels were applied for 45 min. Thus, the following groups were established: (G1) no treatment (negative control/NC); (G2) 35% H2O2 (positive control/PC); (G3) 10% H2O2; (G4) 10% H2O2 + 2 mg/mL MnO2; (G5) 10% H2O2 + 6 mg/mL MnO2; and (G6) 10% H2O2 + 10 mg/mL MnO2. After analyzing bleaching efficacy (ΔE00 and ΔWI), the degradation kinetics of H2O2 and trans-amelodentinal cytotoxicity were determined (n = 8, ANOVA/Tukey; p < 0.05).
Results
G6 presented BE (ΔE00 and ΔWI) statistically similar to G2, which represented conventional in-office bleaching (p = 0.6795; p > 0.9999). A significant reduction in the diffusion of H2O2 occurred in G3, G4, G5, and G6 compared to G2 (p < 0.0001). The highest DK of H2O2 occurred in G6 (p < 0.0001), which had the lowest TC in comparison with all other bleached groups (p ≤ 0.0186).
Conclusion
The addition of 10 mg/mL of MnO2 in a 10% H2O2 bleaching gel potentiates the degradation of this reactive molecule, which increases the BE of the product and decreases TC.
Clinical significance
Replacing a 35% H2O2 gel commonly used for conventional in-office dental bleaching by a 10% H2O2 gel containing 10 mg/mL of MnO2 reduces the cytotoxicity of this professional therapy, maintaining its excellent esthetic efficacy.




Similar content being viewed by others
References
Maran BM, de Paris Matos T, de Castro ADS et al (2020) In-Office bleaching with low/medium vs. high concentrate hydrogen peroxide: a systematic review and meta-analysis. J Dent 103499. https://doi.org/10.1016/j.jdent.2020.103499
Kielbassa AM, Maier M, Gieren AK, Eliav E (2015) Tooth sensitivity during and after vital tooth bleaching: a systematic review on an unsolved problem. Quintessence Int 46(10). https://doi.org/10.3290/j.qi.a34700
Meireles SS, Santos ME, Lustosa ÍMC et al (2021) Effects of a reduced in-office bleaching protocol with 37.5% hydrogen peroxide on effectiveness and tooth sensitivity: a double-blind randomized clinical trial. J Esthet Restor Dent 33(5):824–831. https://doi.org/10.1111/jerd.12744
Rezende M, da Silva KL, Miguel TC et al (2020) Prior application of 10% potassium nitrate to reduce postbleaching sensitivity: a randomized triple-blind clinical trial. J Evid Based Dent Pract 20(2):101406. https://doi.org/10.1016/j.jebdp.2020.101406
Rezende M, Loguercio AD, Kossatz S et al (2016) Predictive factors on the efficacy and risk/intensity of tooth sensitivity of dental bleaching: a multi regression and logistic analysis. J Dent 45:1–6. https://doi.org/10.1016/j.jdent.2015.11.003
Soares DG, Basso FG, Hebling J et al (2014) Concentrations of and application protocols for hydrogen peroxide bleaching gels: effects on pulp cell viability and whitening efficacy. J Dent 42(2):185–198. https://doi.org/10.1016/j.jdent.2013.10.021
Ortecho-Zuta U, de Oliveira Duque CC, de Oliveira Ribeiro RA et al (2021) Polymeric biomaterials maintained the esthetic efficacy and reduced the cytotoxicity of in-office dental bleaching. J Esthet Restor Dent 33(8):1139–1149. https://doi.org/10.1111/jerd.12805
Soares DG, Marcomini N, Duque CCO et al (2019) Increased whitening efficacy and reduced cytotoxicity are achieved by the chemical activation of a highly concentrated hydrogen peroxide bleaching gel. J Appl Oral Sci 27.https://doi.org/10.1590/1678-7757-2018-0453
Soares DG, Basso FG, Hebling J et al (2015) Effect of hydrogen-peroxide-mediated oxidative stress on human dental pulp cells. J Dent 43(6):750–756. https://doi.org/10.1016/j.jdent.2014.12.006
de Souza Costa CA, Riehl H, Kina JF et al (2010) Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109(4):e59–e64. https://doi.org/10.1016/j.tripleo.2009.12.002
Kina JF, Huck C, Riehl H et al (2010) Response of human pulps after professionally applied vital tooth bleaching. Int Endod J 43(7):572–580. https://doi.org/10.1111/j.1365-2591.2010.01713.x
Donassollo SH, Donassollo TA, Coser S et al (2021) Triple-blinded randomized clinical trial comparing efficacy and tooth sensitivity of in-office and at-home bleaching techniques. J Appl Oral Sci 29.https://doi.org/10.1590/1678-7757-2020-0794
Santana MLC, Leal PC, Reis A et al (2019) Effect of anti-inflammatory and analgesic drugs for the prevention of bleaching-induced tooth sensitivity: a systematic review and meta-analysis. J Am Dent Assoc 150(10):818–829. https://doi.org/10.1016/j.adaj.2019.05.004
Ferraz NKL, Nogueira LC, Neiva IM et al (2019) Longevity, effectiveness, safety, and impact on quality of life of low-concentration hydrogen peroxides in-office bleaching: a randomized clinical trial. Clin Oral Investig 23(5):2061–2070. https://doi.org/10.1007/s00784-018-2607-7
Bersezio C, Martín J, Angel P et al (2019) Teeth whitening with 6% hydrogen peroxide and its impact on quality of life: 2 years of follow-up. Odontology 107(1):118–125. https://doi.org/10.1007/s10266-018-0372-3
Al-Omiri MK, Al Nazeh AA, Kielbassa AM et al (2018) Randomized controlled clinical trial on bleaching sensitivity and whitening efficacy of hydrogen peroxide versus combinations of hydrogen peroxide and ozone. Sci Rep 8(1):1–10. https://doi.org/10.1038/s41598-018-20878-0
Al-Omiri MK, Lamfon HA, Nazeh AAA et al (2018) Randomized clinical trial on the comparison of bleaching outcomes using either ozone or hydrogen peroxide. Quintessence Int 49(8). https://doi.org/10.3290/j.qi.a40783
Monterubbianesi R, Tosco V, Bellezze T et al (2021) A comparative evaluation of nanohydroxyapatite-enriched hydrogen peroxide home bleaching system on color, hardness and microstructure of dental enamel. Materials 14(11):3072. https://doi.org/10.3390/ma14113072
Bortolatto JF, Trevisan TC, Bernardi PSI et al (2016) A novel approach for in-office tooth bleaching with 6% H2O2/TiO_N and LED/laser system—a controlled, triple-blinded, randomized clinical trial. Lasers Med Sci 31(3):437–444. https://doi.org/10.1007/s10103-016-1866-2
Yao S, Yuan S, Xu J et al (2006) A hydrogen peroxide sensor based on colloidal MnO2/Na-montmorillonite. Appl Clay Sci 33(1):35–42. https://doi.org/10.1016/j.clay.2006.03.006
Ortecho-Zuta U, de Oliveira Duque CC, Leite ML et al (2019) Effects of enzymatic activation of bleaching gels on hydrogen peroxide degradation rates, bleaching effectiveness, and cytotoxicity. Oper Dent 44(4):414–423. https://doi.org/10.2341/17-276-L
Chen G, Zhao L, Dong YH (2011) Oxidative degradation kinetics and products of chlortetracycline by manganese dioxide. J Hazard Mater 193:128–138. https://doi.org/10.1016/j.jhazmat.2011.07.039
Kuan WH, Hu CY, Liu BS et al (2013) Degradation of antibiotic amoxicillin using 1 × 1 molecular sieve-structured manganese oxide. Environ Technol 34(16):2443–2451. https://doi.org/10.1080/09593330.2013.772658
Prasad AS (2017) Green synthesis of nanocrystalline manganese (II, III) oxide. Mater Sci Semicond Process 71:342–347. https://doi.org/10.1016/j.mssp.2017.08.020
Watts RJ, Sarasa J, Loge FJ et al (2005) Oxidative and reductive pathways in manganese-catalyzed Fenton’s reactions. J Environ Eng 131:158–164. https://doi.org/10.1061/(ASCE)0733-9372(2005)131:1(158)
Choudhary VR, Samanta C, Choudhary TV (2006) Factors influencing decomposition of H2O2 over supported Pd catalyst in aqueous medium. J Mol Catal A Chem 260(1–2):115–120. https://doi.org/10.1016/j.molcata.2006.07.009
Soares DG, Ribeiro APD, da Silveira VF et al (2012) Efficacy and cytotoxicity of a bleaching gel after short application times on dental enamel. Clin Oral Investig 17:1901–1909. https://doi.org/10.1007/s00784-012-0883-1
Suty H, De Traversay C, Cost M (2004) Applications of advanced oxidation processes: present and future. Water Sci Technol 49(4):227–233. https://doi.org/10.2166/wst.2004.0270
Chiam SL, Pung SY, Yeoh FY (2020) Recent developments in MnO2− based photocatalysts for organic dye removal: a review. Environ Sci Pollut Res 27(6):5759–5778. https://doi.org/10.1007/s11356-019-07568-8
Huang M, Xu C, Wu Z et al (2008) Photocatalytic discoloration of methyl orange solution by Pt modified TiO2 loaded on natural zeolite. Dyes Pigm 77(2):327–334. https://doi.org/10.1016/j.dyepig.2007.01.026
de Oliveira Duque CC, Soares DG, Basso FG et al (2017) Influence of enamel/dentin thickness on the toxic and esthetic effects of experimental in-office bleaching protocols. Clin Oral Investig 21(8):2509–2520. https://doi.org/10.1007/s00784-017-2049-7
de Souza Costa CA, Hebling J, Scheffel DL et al (2014) Methods to evaluate and strategies to improve the biocompatibility of dental materials and operative techniques. Dent Mater 30(7):769–784. https://doi.org/10.1016/j.dental.2014.04.010
de Souza Costa CA (2020) Biological aspects of dental materials. J Adhes Dent 22(5):540–544
Cintra LTA, Benetti F, da Silva Facundo AC et al (2013) The number of bleaching sessions influences pulp tissue damage in rat teeth. J Endod 39(12):1576–1580. https://doi.org/10.3290/j.jad.a45409
Sato C, Rodrigues FA, Garcia DM et al (2013) Tooth bleaching increases dentinal protease activity. J Dent Res 92(2):187–192. https://doi.org/10.1177/0022034512470831
Pagano S, Lombardo G, Costanzi E et al (2021) Morpho-functional effects of different universal dental adhesives on human gingival fibroblasts: an in vitro study. Odontology 109(2):524–539. https://doi.org/10.1007/s10266-020-00569-x
Benetti F, Gomes-Filho JE, Ferreira LL et al (2017) Hydrogen peroxide induces cell proliferation and apoptosis in pulp of rats after dental bleaching in vivo: effects of the dental bleaching in pulp. Arch Oral Biol 81:103–109. https://doi.org/10.1016/j.archoralbio.2017.04.013
Soares DG, Basso FG, Scheffel DS et al (2015) Responses of human dental pulp cells after application of a low-concentration bleaching gel to enamel. Arch Oral Biol 60(9):1428–1436. https://doi.org/10.1016/j.archoralbio.2015.06.014
Estay J, Angel P, Bersezio C et al (2020) The change of teeth color, whiteness variations and its psychosocial and self-perception effects when using low vs. high concentration bleaching gels: a one-year follow-up. BMC Oral Health 20(1):1–9. https://doi.org/10.1186/s12903-020-01244-x
Wu M, Hou P, Dong L et al (2019) Manganese dioxide nanosheets: from preparation to biomedical applications. Int J Nanomed 14:4781. https://doi.org/10.2147/IJN.S207666
Layfield RA (2008) Manganese (II): the black sheep of the organometallic family. Chem Soc Rev 37(6):1098–1107. https://doi.org/10.1039/B708850G
Rosa V, Sriram G, McDonald N et al (2022) A critical analysis of research methods and biological experimental models to study pulp regeneration. Int Endod J 00:01–10. https://doi.org/10.1111/iej.13712
Funding
The work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (grants 2020/08882–6 and 2021/01184–4) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grants 302047/2019–0 and 408721/2018–9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study did not need of approval by the Research Ethics Committee involving humans or animals.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira Ribeiro, R.A., Zuta, U.O., Soares, I.P.M. et al. Manganese oxide increases bleaching efficacy and reduces the cytotoxicity of a 10% hydrogen peroxide bleaching gel. Clin Oral Invest 26, 7277–7286 (2022). https://doi.org/10.1007/s00784-022-04688-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-022-04688-3