Abstract
Objectives
Evaluate the modulating effect of ionizing radiation, blood cytokine levels, and bone remodeling of the interface around the implant to understand the radiation mechanisms which can impair the implants receptor site.
Material and methods
Sixty rats were submitted to grade V titanium implants in the femurs and were divided into the following groups: no-irradiation (N-Ir): control group with implant only; early-irradiation (E-Ir): implant + irradiation after 24 h; late-irradiation (L-Ir): implant + irradiation after 4 weeks; and previous-irradiation (P-Ir): irradiation + implant after 4 weeks. The animals in the E-Ir, L-Ir, and P-Ir groups were irradiated in two fractional stages of 15 Gy. At 3 days, 2 weeks, and 7 weeks after the final procedure, five animals were randomly euthanized per group. Serum levels of TNF-ɑ, IL-1β, TGF-β, IL-6, M-CSF, and IL-10 were measured from blood collected prior to euthanasia using the ELISA test. The pieces containing the implants were subjected to immunohistochemical labeling using the tartrate acid resistant to phosphatase, osteocalcin, and caspase-3 markers and mCT. The ANOVA test was used for statistical analysis, and the Tukey multiple comparison test (p < 0.05) was applied.
Results
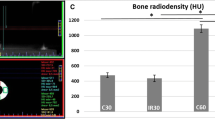
The results indicated that ionizing radiation modifies the production of pro- and anti-inflammatory serum cytokines, the expression of proteins involved in bone remodeling and cellular apoptosis, as well as changes in bone formation.
Conclusions
The results suggests that a longer period between radiotherapy and implant placement surgery when irradiation occurs prior to implant installation would allow the recovery and renewal of bone cells and avoid future failures in osseointegration.
Clinical relevance
The search for modifications caused by ionizing irradiation in bone tissue can indicate the ideal period for implant placement without affecting the osseointegration process.









Similar content being viewed by others
References
Klokkevold PR, Han TJ (2007) How do smoking, diabetes, and periodontitis affect outcomes of implant treatment? Int J Oral Maxillofac Implants 22(suppl):173–202
Chen H, Liu N, Xu X, Qu X, Lu E (2013) Smoking, radiotherapy, diabetes and osteoporosis as risk factors for dental implant failure: a meta-analysis. PLoS One 8:e71955. https://doi.org/10.1371/journal.pone.0071955
Chambrone L, Mandia J, Shibli JA et al (2013) Dental implants installed in irradiated jaws: a systematic review. J Dent Res 92:119–130. https://doi.org/10.1177/0022034513504947
Pellegrino G, Tarsitano A, Ferri A, et al (2018) Long-term results of osseointegrated implant-based dental rehabilitation in oncology patients reconstructed with a fibula free flap. Clin Implant Dent Relat Res 1–8. https://doi.org/10.1111/cid.12658
Mancha De La Plata M, Gas LN, Dez PM et al (2012) Osseointegrated implant rehabilitation of irradiated oral cancer patients. J Oral Maxillofac Surg 70:1052–1063. https://doi.org/10.1016/j.joms.2011.03.032
Pompa G, Saccucci M, Di Carlo G et al (2015) Survival of dental implants in patients with oral cancer treated by surgery and radiotherapy: a retrospective study. BMC Oral Health 15:5. https://doi.org/10.1186/1472-6831-15-5
Verdonck HWD, Meijer GJ, Nieman FH, Stoll C, Riediger D, de Baat C (2008) Quantitative computed tomography bone mineral density measurements in irradiated and non-irradiated minipig alveolar bone: an experimental study. Clin Oral Implants Res 19:465–468. https://doi.org/10.1111/j.1600-0501.2007.01496.x
Hu W-W, Ward BB, Wang Z, Krebsbach PH (2010) Bone regeneration in defects compromised by radiotherapy. J Dent Res 89:77–81. https://doi.org/10.1177/0022034509352151
Chandra A, Lin T, Zhu J, Tong W, Huo Y, Jia H, Zhang Y, Liu XS, Cengel K, Xia B, Qin L (2015) PTH1-34 blocks radiation-induced osteoblast apoptosis by enhancing DNA repair through canonical Wnt pathway. J Biol Chem 290:157–167. https://doi.org/10.1074/jbc.M114.608158
Williams HJ, Davies AM (2006) The effect of X-rays on bone: a pictorial review. Eur Radiol 16:619–633. https://doi.org/10.1007/s00330-005-0010-7
Zou Q, Hong W, Zhou Y, Ding Q, Wang J, Jin W, Gao J, Hua G, Xu X (2016) Bone marrow stem cell dysfunction in radiation-induced abscopal bone loss. J Orthop Surg Res 11:3. https://doi.org/10.1186/s13018-015-0339-9
Lucatto SC, Guilherme A, Dib L et al (2011) Effects of ionizing radiation on bone neoformation: histometric study in Wistar rats tibiae. Acta Cir Bras 26:475–480. https://doi.org/10.1590/s0102-86502011000600012
Da Cunha S, Sarmento VA, Maria L et al (2007) Effects of radiotherapy on bone tissues. Radiol Bras 40:189–192
Vissink A, Jansma J, Spijkervet F et al (2003) Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med 14:199–212
Ohrnel L-O, Brånemark R, Nyman J et al (1997) Effects of irradiation on the biomechanics of osseointegration: an experimental in vivo study in rats. Scand J Plast Reconstr Surg Hand Surg 31:281–293. https://doi.org/10.3109/02844319709008974
Gallet P, Phulpin B, Merlin JL, Leroux A, Bravetti P, Mecellem H, Tran N, Dolivet G (2011) Long-term alterations of cytokines and growth factors expression in irradiated tissues and relation with histological severity scoring. PLoS One 6:1–10. https://doi.org/10.1371/journal.pone.0029399
Barcellos-Hoff MH, Park C, Wright EG (2005) Radiation and the microenvironment - tumorigenesis and therapy. Nat Rev Cancer 5:867–875. https://doi.org/10.1038/nrc1735
Haubner F, Ohmann E, Pohl F, Strutz J, Gassner HG (2012) Wound healing after radiation therapy: review of the literature. Radiat Oncol 7:162. https://doi.org/10.1186/1748-717X-7-162
Lin T, Pajarinen J, Lu L et al (2017) NF-κB as therapeutic target in inflammatory-associated bone diseases. Adv Protein Chem Struct Biol 107:117–154. https://doi.org/10.1016/bs.apcsb.2016.11.002
Katagiri T, Takahashi N (2002) Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis 8:147–159 12108759
Wenxi D, Shufang D, Xiaoling Y, Liming Y (2015) Panax notoginseng saponins suppress radiation-induced osteoporosis by regulating bone formation and resorption. Phytomedicine 22:813–819. https://doi.org/10.1016/j.phymed.2015.05.056
Hong MH, Williams H, Jin CH, Pike JW (2000) The inhibitory effect of interleukin-10 on mouse osteoclast formation involves novel tyrosine-phosphorylated proteins. J Bone Miner Res 15:911–918. https://doi.org/10.1359/jbmr.2000.15.5.911
Yoshitake F, Itoh S, Narita H, Ishihara K, Ebisu S (2008) Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-kB signaling pathways. J Biol Chem 283:11535–11540. https://doi.org/10.1074/jbc.M607999200
Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T (2000) Tumor necrosis factor stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL–RANK interaction. J Exp Med 191:275–285
Shiratori T, Kyumoto-Nakamura Y, Kukita A, Uehara N, Zhang J, Koda K, Kamiya M, Badawy T, Tomoda E, Xu X, Yamaza T, Urano Y, Koyano K, Kukita T (2018) IL-1β induces pathologically activated osteoclasts bearing extremely high levels of resorbing activity: a possible pathological subpopulation of osteoclasts, accompanied by suppressed expression of kindlin-3 and talin-1. J Immunol 200:218–228. https://doi.org/10.4049/jimmunol.1602035
Wijekoon S, Bwalya E, Fang J et al (2017) Chronological differential effects of pro-inflammatory cytokines on RANKL-induced osteoclast differentiation of canine bone marrow-derived macrophages. J Vet Med Sci 79:2030–2035
Morony S, Capparelli C, Lee R, Shimamoto G, Boone T, Lacey DL, Dunstan CR (1999) A chimeric form of osteoprotegerin inhibits hypercalcemia and bone resorption induced by IL-1β,TNF-α, PTH, PTHrP, and 1,25(OH)2D3. J Bone Miner Res 14:1478–1485
Takayanagi H (2007) Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. J Clin Immunol 7:292–304. https://doi.org/10.1007/s10875-009-9316-6
Sakakeeny MA, Harrington M, Leif J et al (1994) Effects of gamma-irradiation on the M-CSF-promoter linked to a chloramphenicol aminoacyl transferase reporter gene expressed in a clonal murine bone marrow stromal cell line. Stem Cells 12:87–94. https://doi.org/10.1002/stem.5530120115
Hodge JM, Kirkland MA, Nicholson GC (2007) Multiple roles of M-CSF in human osteoclastogenesis. J Cell Biochem 102:759–768. https://doi.org/10.1016/j.bbrc.2004.06.097
Rupnow BA, Knox SJ (1999) The role of radiation-induced apoptosis as a determinant of tumor responses to radiation therapy. Apoptosis 4:115–143. https://doi.org/10.1023/A:1009675028784
Rahmanian N, Hosseinimehr SJ, Khalaj A (2016) The paradox role of caspase cascade in ionizing radiation therapy. J Biomed Sci 23:1–13. https://doi.org/10.1186/s12929-016-0306-8
Michelin S, Perez MDR, Dubner D, Gisone P (2004) Increased activity and involvement of caspase-3 in radiation-induced apoptosis in neural cells precursors from developing rat brain. Neurotoxicology 25:387–398. https://doi.org/10.1016/j.neuro.2003.08.009
De Vasconcellos LMR, Barbara MAM, Deco CP et al (2014) Healing of normal and osteopenic bone with titanium implant and low-level laser therapy (GaAlAs): a histomorphometric study in rats. Lasers Med Sci 29:575–580. https://doi.org/10.1007/s10103-013-1326-1
de Vasconcellos LMR, Barbara MAM, da Silva RE et al (2016) Titanium scaffold osteogenesis in healthy and osteoporotic rats is improved by the use of low-level laser therapy (GaAlAs). Lasers Med Sci 31:899–905. https://doi.org/10.1007/s10103-016-1930-y
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman D (2013) Improving bioscience research reporting: the arrive guidelines for reporting animal research. Animals 4:35–44. https://doi.org/10.3390/ani4010035
Quinn R (2005) Comparing rat’s to human’s age: how old is my rat in people years? Nutrition 21:775–777. https://doi.org/10.1016/j.nut.2005.04.002
Ocaña RP, Rabelo GD, Sassi LM, Rodrigues VP, Alves FA (2017) Implant osseointegration in irradiated bone: an experimental study. J Periodontal Res 52:505–511. https://doi.org/10.1111/jre.12416
da Cunha S, Sarmento V, Ramalho L et al (2007) Effects of radiotherapy on bone tissue. Radiol Bras 40:189–192. https://doi.org/10.1590/S0100-39842007000300011
dos Santos P, de Molon RS, Queiroz TP et al (2016) Evaluation of bone substitutes for treatment of peri-implant bone defects: biomechanical, histological, and immunohistochemical analyses in the rabbit tibia. J Periodontal Implant Sci 46:176–196. https://doi.org/10.5051/jpis.2016.46.3.176
dos Santos PL, Queiroz TP, Margonar R, Gomes de Souza Carvalho AC, Okamoto R, de Souza Faloni AP, Garcia Júnior IR (2013) Guided implant surgery: what is the influence of this new technique on bone cell viability? J Oral Maxillofac Surg 71:505–512. https://doi.org/10.1016/j.joms.2012.10.017
Queiroz TP, Souza FÁ, Okamoto R, Margonar R, Pereira-Filho VA, Garcia Júnior IR, Vieira EH (2008) Evaluation of immediate bone-cell viability and of drill wear after implant osteotomies: immunohistochemistry and scanning electron microscopy analysis. J Oral Maxillofac Surg 66:1233–1240. https://doi.org/10.1016/j.joms.2007.12.037
Esteves J, Marcantonio E Jr, Faloni A et al (2013) Dynamics of bone healing after osteotomy with piezosurgery or conventional drilling – histomorphometrical, immunohistochemical, and molecular analysis. J Transl Med 11:221. https://doi.org/10.1186/1479-5876-11-221
Leventis M, Fairbairn P, Mangham C, Galanos A, Vasiliadis O, Papavasileiou D, Horowitz R (2018) Bone healing in rabbit calvaria defects using a synthetic bone substitute: a histological and micro-CT comparative study. Materials (Basel) 11 https://doi.org/10.3390/ma11102004
Terheyden H, Lang NP, Bierbaum S, Stadlinger B (2012) Osseointegration - communication of cells. Clin Oral Implants Res 23:1127–1135. https://doi.org/10.1111/j.1600-0501.2011.02327.x
Trindade R, Albrektsson T, Galli S, Prgomet Z, Tengvall P, Wennerberg A (2018) Osseointegration and foreign body reaction: titanium implants activate the immune system and suppress bone resorption during the first 4 weeks after implantation. Clin Implant Dent Relat Res 20:82–91. https://doi.org/10.1111/cid.12578
Okamoto K, Nakashima T, Shinohara M, Negishi-Koga T, Komatsu N, Terashima A, Sawa S, Nitta T, Takayanagi H (2017) Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol Rev 97:1295–1349. https://doi.org/10.1152/physrev.00036.2016
Theill LE, Boyle WJ, Penninger JM (2002) RANK-L AND RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol 20:795–823. https://doi.org/10.1146/annurev.immunol.20.100301.064753
Maridas DE, Rendina-Ruedy E, Le PT, Rosen CJ (2018) Isolation, culture, and differentiation of bone marrow stromal cells and osteoclast progenitors from mice. J Vis Exp. https://doi.org/10.3791/56750
Raggatt LJ, Partridge NC (2010) Cellular and molecular mechanisms of bone remodeling. J Biol Chem 285:25103–25108. https://doi.org/10.1074/jbc.R109.041087
Kirstein B, Chambers TJ, Fuller K (2006) Secretion of tartrate-resistant acid phosphatase by osteoclasts correlates with resorptive behavior. J Cell Biochem 98:1085–1094. https://doi.org/10.1002/jcb.20835
Huang RL, Sun Y, Ho CK, Liu K, Tang QQ, Xie Y, Li Q (2018) IL-6 potentiates BMP-2-induced osteogenesis and adipogenesis via two different BMPR1A-mediated pathways article. Cell Death Dis 9:144. https://doi.org/10.1038/s41419-017-0126-0
Lampiasi N, Russo R, Zito F (2016) The alternative faces of macrophage generate osteoclasts. Biomed Res Int 2016:9. https://doi.org/10.1155/2016/9089610
Abbas AK, Lichtman AH, Pilai S (2015) Imunologia celular e molecular, 8a. Rio de Janeiro
Moghaddam AS, Mohammadian S, Vazini H et al (2018) Macrophage plasticity, polarization and function in health and disease
Pixley FJ, Stanley ER (2004) CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol 14:628–638. https://doi.org/10.1016/j.tcb.2004.09.016
Saraiva GL, Lazaretti-Castro M (2002) Biochemical bone markers in clinical practice. Brazilian Arch Endocrinol Metab 46:72–78. https://doi.org/10.1590/S0004-27302002000100010
Sawajiri M, Mizoe J (2003) Changes in bone volume after irradiation with carbon ions. Radiat Environ Biophys 42:101–106. https://doi.org/10.1007/s00411-003-0191-x
Doh R-M, Kim S, Keum KC, Kim JW, Shim JS, Jung HS, Park KM, Chung MK (2016) Postoperative irradiation after implant placement: a pilot study for prosthetic reconstruction. J Adv Prosthodont 363:363–371. https://doi.org/10.4047/jap.2016.8.5.363
Flores-Ruiz R, Castellanos-Cosano L, Serrera-Figallo M-A, Cano-Diaz E, Torres-Lagares D, Gutierrez-Perez JL (2018) Implant survival in patients with oral cancer: a 5-year follow-up. J Clin Exp Dent 10:603–612. https://doi.org/10.4317/jced.54937
Chrcanovic B, Albrektsson T, Wennerberg A (2016) Dental implants in irradiated versus nonirradiated patients: a meta-analysis. Head Neck 1–14. https://doi.org/10.1002/hed.23875
Bolind P, Johansson CB, Johansson P, Granstrom G, Albrektsson T (2006) Retrieved implants from irradiated sites in humans: a histologic/histomorphometric investigation of oral and craniofacial implants. Clin Implant Dent Relat Res 8:142–150. https://doi.org/10.1111/j.1708-8208.2006.00010.x
Brasseur M, Brogniez V, Grégoire V, Reychler H, Lengelé B, D’Hoore W, Nyssen-Behets C (2006) Effects of irradiation on bone remodelling around mandibular implants: an experimental study in dogs. Int J Oral Maxillofac Surg 35:850–855. https://doi.org/10.1016/j.ijom.2006.03.016
Acknowledgements
The authors are thankful to Dr. Odair Lelis Gonçalez and all of the Institute of Advanced Studies technical team, Sao Jose dos Campos, Sao Paulo, Brazil for assistance during irradiation of the rats. The authors are also thankful for technical support by Mr. Walter Cruz for his help with histhological procedures and tissue preparations and the Department of Bioscience and Diagnosis team, Sao Jose dos Campos, Sao Paulo, Brazil. We are extremely grateful to Emfils Comércio Produtos Odontológicos® for the free donation of the implants used in this study.
Funding
This work was supported by the Foundation for Research Support of the State of Sao Paulo, Sao Paulo, Brazil. Process 2015/24986-8, 2016/19707-5, 2017/00543-5, and 2017/04389-0.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This project was approved by the Animal Ethics Committee (CEUA, Protocol 003/2016) of the Institute of Science and Technology of the Campus of Sao Jose dos Campos/UNESP and was carried out in accordance with the ethical principles adopted by the Brazilian National Animal Care Ethical Council (CONCEA).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Cruz Vegian, M.R., Costa, B.C.A., de Fátima Santana-Melo, G. et al. Systemic and local effects of radiotherapy: an experimental study on implants placed in rats. Clin Oral Invest 24, 785–797 (2020). https://doi.org/10.1007/s00784-019-02946-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-02946-5