Abstract
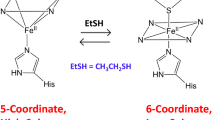
A series of ferric and ferrous derivatives of wild-type ascorbate peroxidase (APX) and of an engineered K+-site mutant of APX that has had its potassium cation binding site removed have been examined by electronic absorption and magnetic circular dichroism (MCD) spectroscopy at 4 °C. Wild-type ferric APX has spectroscopic properties that are very similar to those of ferric cytochrome c peroxidase (CCP) and likely exists primarily as a five-coordinate high-spin heme ligated on the proximal side by a histidine at pH 7. There is also evidence for minority contributions from six-coordinate high- and low-spin species (histidine-water, histidine-hydroxide, and bis-histidine). The K+-site mutant of APX varies considerably in the electronic absorption and MCD spectra in both the ferric and ferrous states when compared with spectra of the wild-type APX. The electronic absorption and MCD spectra of the engineered K+-site APX mutant are essentially identical to those of cytochrome b 5, a known bis-imidazole (histidine) ligated heme system. It therefore appears that the K+-site mutant of APX has undergone a conformational change to yield a bis-histidine coordination structure in both the ferric and ferrous oxidation states at neutral pH. This conformational change is the result of mutagenesis of the protein to remove the K+-binding site which is located ∼8 Å from the peroxide binding pocket. Thus, mutations of protein residues on the proximal side of the heme cause changes in iron ligation on the distal side.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 7 August 1998 / Accepted: 2 December 1998
Rights and permissions
About this article
Cite this article
Cheek, J., Mandelman, D., Poulos, T. et al. A study of the K+-site mutant of ascorbate peroxidase: mutations of protein residues on the proximal side of the heme cause changes in iron ligation on the distal side. JBIC 4, 64–72 (1999). https://doi.org/10.1007/s007750050290
Issue Date:
DOI: https://doi.org/10.1007/s007750050290