Abstract
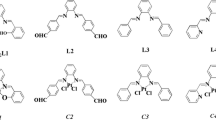
Four new complexes of Pt(II) and Pd(II), [Pd(L1)Cl]Cl 1, [Pd(L2)Cl]Cl 2, [Pt(L1)Cl]Cl 3 and [Pt(L2)Cl]Cl 4 (where L1 = 2,6-bis(5,6-diphenyl-1,2,4-triazin-3-yl)pyridine and L2 = 2,6-bis(5,6-dipropyl-1,2,4-triazin-3-yl)pyridine), were synthesized. Characterization of the complexes was performed using elemental analysis, IR, 1H NMR spectroscopy and MALDI-TOF mass spectrometry. The substitution reactions of 1–4 complexes with l-methionine (l-met), l-cysteine (l-cys) and guanosine-5ʹ-monophosphate (5ʹ-GMP), were studied spectrophotometrically at physiological conditions. Complexes with ligand L1 (1 or 3) were more reactive than those with ligand L2 (2 or 4) by a factor ranging up to 1.57 and 3.71, respectively. The order of reactivity of the nucleophiles was: l-met > l-cys > 5ʹ-GMP. The interactions of complexes with calf thymus-DNA (CT-DNA) and human serum albumin (HSA) were studied by Uv–Vis absorption and fluorescence emission spectroscopy. Competitive binding studies with intercalative agent ethidium bromide (EB) and minor groove binder Hoechst 33258 were performed as well. All studied complexes can interact with DNA through the intercalation and minor groove binding, where the latter was preferred. The binding constants (103 and 104 M−1) for the interaction of complexes with HSA indicate the moderate binding affinity of complexes 1–4 to protein. The trends in the experimental results of binding studies between complexes 3 and 4 with DNA and HSA were compared to those obtained from the molecular docking study. Biological evaluation of cytotoxicity of 1 and 2 on HCT-116 and MDA-MB-231 cell lines showed significant cytotoxic and prooxidative character, while 2 also exerted extraordinary selectivity towards colon cancer in comparison to breast cancer cells.
Graphic abstract
The nucleophilic substitution reactions, DNA/HSA interactions, molecular docking and biological activity of bis(triazinyl)pyridine complexes of Pt(II) and Pd(II) were studied.






Similar content being viewed by others
Abbreviations
- L1:
-
2,6-Bis(5,6-diphenyl-1,2,4-triazin-3-yl)pyridine
- L2:
-
2,6-Bis(5,6-dipropyl-1,2,4-triazin-3-yl)pyridine
- L-met:
-
L-methionine
- L-cys:
-
L-cysteine
- 5′-GMP:
-
Guanosine-5′-monophosphate
- CT-DNA:
-
Calf thymus-DNA
- HAS:
-
Human serum albumin
- EB:
-
Ethidium bromide
- Hoechst 33258:
-
2-(4-hydroxyphenyl)-5-[5-(4-methylpipera-zine-1-yl)-benzimidazo-2-yl]-benzimidazole
- DMF:
-
Dimethylformamide
- HCT-116:
-
Human colorectal carcinoma cells
- MDA-MB-231:
-
Human breast cancer cells
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Int J Cancer 136:E359-386
Alessio E (2011) Bioinorganic medicinal chemistry. Wiley-VCH Verlag & Co KGaA, Weinheim, Germany
Anthony JE, Bolitho EM, Bridgewater HE, Carter OWL, Donnelly JM, Imberti C, Lant EC, Lermyte F, Needham RJ, Palau M, Sadler PJ, Shi H, Wang FX, Zhang WY, Zhang Z (2020) Chem Sci 11:12888–12917
Simović AR, Bogojeski J, Petrović B, Stević SJ (2020) Macroheterocycles 13:201–209
Petrović B, Jovanović S, Puchta R, van Eldik R (2019) Inorg Chim Acta 495:118953
Ndagi U, Mhlongo N, Soliman ME (2017) Drug Des Devel Ther 11:599–616
Fanelli M, Formica M, Fusi V, Giorgi L, Micheloni M, Paoli P (2016) Coord Chem Rev 310:41–79
Kritchenkov AS, Stanishevskii YM, Skorik YZ (2019) Pharm Chem J 53:8–16
Wang D, Lippard SJ (2005) Nat Rev Drug Discov 4:307–320
Aldossary SA (2019) Biomed Pharmacol J 12:7–15
Arsenijević M, Milovanović M, Jovanović S, Arsenijević N, Simović Marković B, Gazdić M, Volarević V (2017) J Biol Inorg Chem 22:807–817
Ćoćić D, Jovanović-Stević S, Jelić R, Matić S, Popović S, Djurdjević P, Baskić D, Petrović P (2020) Dalton Trans 49:14411–14431
Ćoćić D, Jovanović S, Radisavljević S, Korzekwa J, Scheurer A, Puchta R, Baskić D, Todorović D, Popović S, Matić S, Petrović B (2018) J Inorg Biochem 189:91–102
Jovanović S, Obrenčević K, Bugarčić ŽD, Popović I, Žakula J, Petrović B (2016) Dalton Trans 45:12444–12457
Bugarčić ŽD, Bogojeski J, Petrović B, Hochreuther S, van Eldik R (2012) Dalton Trans 41:12329–12345
Petrović B, Bugarčić ŽD, Dees A, Ivanović-Burmazović I, Heinemann F, Puchta R, Steinmann SN, Corminboeuf C, van Eldik R (2012) Inorg Chem 51:1516–1529
Omondi OR, Ojwacha SO, Jaganyi D (2020) Inorg Chim Acta 512:119883
Timerbaev AR, Hartinger CG, Aleksenko SS, Keppler BK (2006) Chem Rev 106:2224–2248
Case FH (1971) J Heterocycl Chem 8:1043–1046
Kolarik Z, Müllich U, Gassner F (1999) Solvent Extr Ion Exch 17:1155–1170
Shahabadi N, Maghsudi M (2014) Mol Biosyst 10:338–347
Dimiza F, Fountoulaki S, Papadopoulos AN, Kontogiorgis CA, Tangoulis V, Raptopoulou CP, Psycharis V, Terzis A, Kessissoglou DP, Psomas G (2011) Dalton Trans 40:8555–8568
Becke AD (1993) J Phys Chem 97:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Stephens PJ, Devli FJ, Chabalowski CF, Frisch MJ (1994) J Phys Chem 98:11623–11627
Andrae D, Häußermann U, Dolg M, Stoll H, Preuß H (1990) Theor Chim Acta 77:123–141
Weigend F, Ahlrichs R (2005) Phys Chem Chem Phys 7:3297–3305
Gaussian 09, Revision C.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian, Inc, Wallingford CT
Thomsen R, Christensen MH (2006) J Med Chem 49:3315–3321
Radisavljević S, Ćoćić D, Jovanović S, Šmit B, Petković M, Milivojević N, Planojević N, Marković S, Petrović B (2019) J Biol Inorg Chem 24:1057–1076
Medjedović M, Rilak-Simović A, Ćoćić D, Milutinović M, Senft L, Blagojević S, Milivojević N, Petrović B (2020) Polyhedron 178:114334–114344
Petrović A, Živanović M, Puchta R, Ćoćić D, Scheurer A, Milivojevic N, Bogojeski J (2020) Dalton Trans 49:9070–9085
Radisavljević S, Đeković-Kesić A, Ćoćić D, Puchta R, Senft L, Milutinović M, Milivojević N, Petrović B (2020) New J Chem 44:11172–11187
Petrović AZ, Ćoćić D, Bockfeld D, Živanović M, Milivojević N, Virijević K, Janković N, Scheurer A, Vraneš M, Bogojeski JV (2021) Inorg Chem Front 8:2749–2770
Petrović A, Milutinović MM, Petri ET, Živanović M, Milivojević N, Puchta R, Scheurer A, Korzekwa J, Klisurić OR, Bogojeski J (2019) Inorg Chem 58:307–319
Živković MD, Rajković S, Glišić BÐ, Drašković NS, Djuran MI (2017) Bioorg Chem 72:190–198
Bugarčić ŽD, Bogojeski J, van Eldik R (2015) Coord Chem Rev 292:91–106
Rizvi MA, Zaki M, Afzal MM, Mane M, Kumar M, Shah BA, Srivastav S, Srikrishna S, Peerzada GM, Tabassum S (2015) Eur J Med Chem 90:876–888
Milutinović MM, Bogojeski JV, Klisurić O, Scheurer A, Elmroth SKC, Bugarčić ŽD (2016) Dalton Trans 45:15481–15491
Recio Despaigne AA, Da Silva JG, da Costa PR, dos Santos RG, Beraldo H (2014) Molecules 19:17202–17220
Boger BC, Fink BE, Brunette SR, Tse WC, Hedrick MP (2001) J Am Chem Soc 123:5878–5891
Fornander LH, Wu L, Billeter M, Lincoln P, Nordén B (2013) J Phys Chem B 117:5820–5830
Novakova O, Chen H, Vrana O, Rodger A, Sadler PJ, Brabec V (2003) Biochemistry 42:11544–11554
Wang F, Huang W, Dai ZX (2008) J Mol Struct 875:509–514
Kljun J, Bratsos I, Alessio E, Psomas G, Repnik U, Butinar M, Turk B, Turel I (2013) Inorg Chem 52:9039–9052
Afanasev IB (2009) Signaling mechanisms of oxygen and nitrogen free radicals. CRC Press, Inc, Boca Raton
Acknowledgements
The authors gratefully acknowledge financial support from the Ministry of Education, Science and Technological Development of the Republic of Serbia (Agreement No. 451-03-9/2021-14/200378 and Agreement No. 451-03-9/2021-14/200122). MALDI-TOF mass spectrometry was conducted by Research Professor Dr. Marijana Petković at CQM-Madeira Chemistry Research Centre, University of Madeira, Funchal, Portugal, supported by the FCT-Fundação para a Ciência e a Tecnologia (CQM Base Fund—UIDB/00674/2020, and Programmatic Fund—UIDP/00674/2020), Madeira 14-20 Program (project PROEQUIPRAM-Reforço do Investimento em Equipamentos e Infrastructures Científcas na RAM-M1420-01-0145-FEDER-000008) and by ARDITI-Agência Regional para o Desenvolvimento da InvestigaçãoTecnologia e Inovação, ARDITI-CQM_2019-018-ISG.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. SJ-S: supervision, validation, writing—review, and editing; SR: investigation; AS: investigation, methodology; DĆ: investigation, software; BŠ: formal analysis; MP: formal analysis; MNŽ: investigation, methodology; KV: investigation; BP: investigation, resources, methodology. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jovanović-Stević, S., Radisavljević, S., Scheurer, A. et al. Bis(triazinyl)pyridine complexes of Pt(II) and Pd(II): studies of the nucleophilic substitution reactions, DNA/HSA interactions, molecular docking and biological activity. J Biol Inorg Chem 26, 625–637 (2021). https://doi.org/10.1007/s00775-021-01879-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-021-01879-3