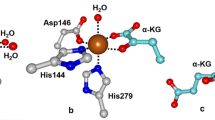
Abstract
Iron(II)-containing homoprotocatechuate 2,3-dioxygenase (FeHPCD) activates O2 to catalyze the aromatic ring opening of homoprotocatechuate (HPCA). The enzyme requires FeII for catalysis, but MnII can be substituted (MnHPCD) with essentially no change in the steady-state kinetic parameters. Near simultaneous O2 and HPCA activation has been proposed to occur through transfer of an electron or electrons from HPCA to O2 through the divalent metal. In O2 reactions with MnHPCD–HPCA and the 4-nitrocatechol (4NC) complex of the His200Asn (H200N) variant of FeHPCD, this transfer has resulted in the detection of a transient MIII–O2 ·− species that is not observed during turnover of the wild-type FeHPCD. The factors governing formation of the MIII–O2 ·− species are explored here by EPR spectroscopy using MnHPCD and nitric oxide (NO) as an O2 surrogate. Both the HPCA and the dihydroxymandelic substrate complexes of MnHPCD bind NO, thus representing the first reported stable MnNO complexes of a nonheme enzyme. In contrast, the free enzyme, the MnHPCD–4NC complex, and the MnH200N and MnH200Q variants with or without HPCA bound do not bind NO. The MnHPCD–ligand complexes that bind NO are also active in normal O2-linked turnover, whereas the others are inactive. Past studies have shown that FeHPCD and the analogous variants and catecholic ligand complexes all bind NO, and are active in normal turnover. This contrasting behavior may stem from the ability of the enzyme to maintain the approximately 0.8-V difference in the solution redox potentials of FeII and MnII. Owing to the higher potential of Mn, the formation of the NO adduct or the O2 adduct requires both strong charge donation from the bound catecholic ligand and additional stabilization by interaction with the active-site His200. The same nonoptimal electronic and structural forces that prevent NO and O2 binding in MnHPCD variants may lead to inefficient electron transfer from the catecholic substrate to the metal center in variants of FeHPCD during O2-linked turnover. Accordingly, past studies have shown that intermediate FeIII species are observed for these mutant enzymes.








Similar content being viewed by others
Abbreviations
- 4NC:
-
4-Nitrocatechol
- DEA NONOate:
-
Diethylamine NONOate diethylammonium salt
- DHM:
-
Dihydroxymandelic acid
- EPR:
-
Electron paramagnetic resonance
- FeHPCD:
-
Homoprotocatechuate 2,3-dioxygenase from Brevibacterium fuscum
- HPCA:
-
Homoprotocatechuate
- HPCD:
-
Homoprotocatechuate 2,3-dioxygenase
- ICP-AES:
-
Inductively coupled plasma atomic emission spectroscopy
- MnHPCD:
-
Homoprotocatechuate 2,3-dioxygenase from Brevibacterium fuscum in which the FeII is replaced by MnII
- RFQ:
-
Rapid freeze quench
References
Lipscomb JD (2008) Curr Opin Struct Biol 18:644–649
Koehntop KD, Emerson JP, Que L (2005) J Biol Inorg Chem 10:87–93
Kovaleva EG, Lipscomb JD (2008) Nat Chem Biol 4:186–193. doi:10.1038/nchembio.71
Vetting MW, Wackett LP, Que L Jr, Lipscomb JD, Ohlendorf DH (2004) J Bacteriol 186:1945–1958
Wang YZ, Lipscomb JD (1997) Protein Expr Purif 10:1–9. doi:10.1006/prep.1996.0703
Boldt YR, Sadowsky MJ, Ellis LBM, Que L Jr, Wackett LP (1995) J Bacteriol 177:1225–1232
Emerson JP, Kovaleva EG, Farquhar ER, Lipscomb JD, Que L Jr (2008) Proc Natl Acad Sci USA 105:7347–7352
Fielding AJ, Kovaleva EG, Farquhar ER, Lipscomb JD, Que L Jr (2011) J Biol Inorg Chem 16:341–355. doi:10.1007/s00775-010-0732-0
Mbughuni MM, Chakrabarti M, Hayden JA, Meier KK, Dalluge JJ, Hendrich MP, Münck E, Lipscomb JD (2011) Biochemistry 50:10262–10274. doi:10.1021/bi201436n
Gunderson WA, Zatsman AI, Emerson JP, Farquhar ER, Que L Jr, Lipscomb JD, Hendrich MP (2008) J Am Chem Soc 130:14465–14467. doi:10.1021/ja8052255
Mbughuni MM, Chakrabarti M, Hayden JA, Bominaar EL, Hendrich MP, Münck E, Lipscomb JD (2010) Proc Natl Acad Sci USA 107:16788–16793
Arciero DM, Lipscomb JD, Huynh Boi H, Kent TA, Münck E (1983) J Biol Chem 258:14981–14989
Ford PC, Lorkovic IM (2002) Chem Rev 102:993–1018
Groce SL, Lipscomb JD (2005) Biochemistry 44:7175–7188. doi:10.1021/bi050180v
Whiting AK, Boldt YR, Hendrich MP, Wackett LP, Que L Jr (1996) Biochemistry 35:160–170
Krzystek J, Ozarowski A, Telser J (2006) Coord Chem Rev 250:2308–2324. doi:10.1016/j.ccr.2006.03.016
Zheng M, Khangulov SV, Dismukes GC, Barynin VV (1994) Inorg Chem 33:382–387
Peloquin JM, Campbell KA, Randall DW, Evanchik MA, Pecoraro VL, Armstrong WH, Britt RD (2000) J Am Chem Soc 122:10926–10942
Campbell KA, Yikilmaz E, Grant CV, Gregor W, Miller A-F, Britt RD (1999) J Am Chem Soc 121:4714–4715
Miller MA, Lipscomb JD (1996) J Biol Chem 271:5524–5535
Arciero DM, Orville AM, Lipscomb JD (1985) J Biol Chem 260:14035–14044
Gelb MH, Toscano WA Jr, Sligar SG (1982) Proc Natl Acad Sci USA 79:5758–5762
Ioannidis N, Schansker G, Barynin VV, Petrouleas V (2000) J Biol Inorg Chem 5:354–363
Franz KJ, Lippard SJ (1998) J Am Chem Soc 120:9034–9040
Merkle AC, Fry NL, Mascharak PK, Lehnert N (2011) Inorg Chem 50:12192–12203. doi:10.1021/ic201967f
Taguchi T, Gupta R, Lassalle-Kaiser B, Boyce DW, Yachandra VK, Tolman WB, Yano J, Hendrich MP, Borovik AS (2012) J Am Chem Soc 134:1996–1999. doi:10.1021/ja210957u
Tangen E, Conradie J, Franz K, Friedle S, Telser J, Lippard SJ, Ghosh A (2010) Inorg Chem 49:2701–2705
Brown CA, Pavlosky MA, Westre TE, Zhang Y, Hedman B, Hodgson KO, Solomon EI (1995) J Am Chem Soc 117:715–732
Serres RG, Grapperhaus CA, Bothe E, Bill E, Weyhermuller T, Neese F, Wieghardt K (2004) J Am Chem Soc 126:5138–5153. doi:10.1021/ja030645+
Duboc C, Collomb M-N, Neese F (2010) Appl Magn Reson 37:229–245
Gatjens J, Sjodin M, Pecoraro VL, Un S (2007) J Am Chem Soc 129:13825–13827
Un S, Tabares LC, Cortez Ns, Hiraoka BY, Yamakura F (2004) J Am Chem Soc 126:2720–2726
Vaillancourt FH, Barbosa CJ, Spiro TG, Bolin JT, Blades MW, Turner RF, Eltis LD (2002) J Am Chem Soc 124:2485–2496
Shu L, Chiou Y-M, Orville AM, Miller MA, Lipscomb JD, Que L Jr (1995) Biochemistry 34:6649–6659
Reynolds MF, Costas M, Ito M, Jo D-H, Tipton AA, Whiting AK, Que L (2003) J Biol Inorg Chem 8:263–272
Bugg TD, Ramaswamy S (2008) Curr Opin Chem Biol 12:134–140. doi:10.1016/j.cbpa.2007.12.007
Vaillancourt FH, Bolin JT, Eltis LD (2006) Crit Rev Biochem Mol Biol 41:241–267
Costas M, Mehn MP, Jensen MP, Que L Jr (2004) Chem Rev 104:939–986. doi:10.1021/cr020628n
Boldt YR, Whiting AK, Wagner ML, Sadowsky MJ, Que L Jr, Wackett LP (1997) Biochemistry 36:2147–2153
Fielding AJ, Lipscomb JD, Que L Jr (2012) J Am Chem Soc 134:796–799. doi:10.1021/ja2095365
Acknowledgments
This work was supported by grants GM24689 (to J.D.L), GM33162 (to L.Q.), and GM77387 (to M.P.H.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayden, J.A., Farquhar, E.R., Que, L. et al. NO binding to Mn-substituted homoprotocatechuate 2,3-dioxygenase: relationship to O2 reactivity. J Biol Inorg Chem 18, 717–728 (2013). https://doi.org/10.1007/s00775-013-1016-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-013-1016-2