Abstract
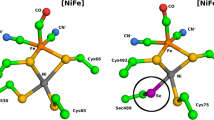
The kinetics of the activation and anaerobic inactivation processes of Desulfovibrio gigas hydrogenase have been measured in D2O by FTIR spectroelectrochemistry. A primary kinetic solvent isotope effect was observed for the inactivation process but not for the activation step. The kinetics of these processes have been also measured after replacement of a glutamic residue placed near the active site of an analogous [NiFe] hydrogenase from Desulfovibrio fructosovorans. Its replacement by a glutamine affected greatly the kinetics of the inactivation process but only slightly the activation process. The interpretation of the experimental results is that the rate-limiting step for anaerobic inactivation is the formation from water of a μ-OH− bridge at the hydrogenase active site, and that Glu25 has a role in this step.







Similar content being viewed by others
References
Cammack R (2001) In: Cammack R, Frey M, Robson R (eds) Hydrogen as a fuel. Taylor & Francis, London, pp 159–180
Volbeda A, Charon MH, Piras C, Hatchikian EC, Frey M, Fontecilla-Camps JC (1995) Nature 373:580–587
Volbeda A, Garcin E, Piras C, De Lacey AL, Fernandez VM, Hatchikian EC, Frey M, Fontecilla-Camps JC (1996) J Am Chem Soc 118:12989–12996
Bagley KA, Van Garderen CJ, Chen M, Duin EC, Albracht SPJ, Woodruff WH (1994) Biochemistry 33:9229–9236
Bagley KA, Duin EC, Roseboom W, Albracht SPJ, Woodruff WH (1995) Biochemistry 34:5527–5535
Happe RP, Roseboom W, Pierik AJ, Albracht SPJ, Bagley KA (1997) Nature 385:126
Higuchi Y, Ogata H, Miki K, Yasuoka N, Yagi T (1999) Structure 7:549–556
Garcin E, Vernede X, Hatchikian EC, Volbeda A, Frey M, Fontecilla-Camps JC (1999) Structure 7:557–566
Fernandez VM, Hatchikian EC, Cammack R (1985) Biochim Biophys Acta 832:69–79
Moura JJG, Moura I, Huyhn BH, Kruger HJ, Teixeira M, DuVarney RC, DerVartanian DV, Xavier AV, Peck HD Jr, LeGall J (1982) Biochem Biophys Res Commun 108:1388–1393
Fernandez VM, Hatchikian EC, Patil DS, Cammack R (1986) Biochim Biophys Acta 883:145–154
Teixeira M, Moura I, Xavier AV, Huyhn BH, DerVartanian DV, Peck HD Jr, LeGall J, Moura JJG (1985) J Biol Chem 260:8942–8950
Cammack R, Patil DS, Hatchikian EC, Fernandez VM (1987) Biochim Biophys Acta 912:98–109
Roberts LM, Lindahl PA (1995) J Am Chem Soc 117:2565–2572
De Lacey AL, Hatchikian EC, Volbeda A, Frey M, Fontecilla-Camps JC, Fernandez VM (1997) J Am Chem Soc 119:7181–7189
Coremans JMCC, van der Zwaan JW, Albracht SPJ (1992) Biochim Biophys Acta 1119:157–168
Mege RM, Bourdillon C (1985) J Biol Chem 260:14701–14706
Jones AK, Lamle SE, Pershad HR, Vincent KA, Albracht SPJ, Armstrong FA (2003) J Am Chem Soc 125:8505–8514
Van der Zwaan JW, Coremans JMCC, Bouwens ECM, Albracht SPJ (1990) Biochim Biophys Acta 1041:101–110
Davidson G, Choudhury SB, Gu Z, Bose K, Roseboom W, Albracht SPJ, Maroney MJ (2000) Biochemistry 39:7468–7479
Carepo M, Tierney DL, Brondino CD, Yang TC, Pamplona A, Telser J, Moura I, Moura JJG, Hoffman BM (2002) J Am Chem Soc 124:281–286
Fan HJ, Hall MB (2001) J Biol Inorg Chem 6:467–473
Stein M, Lubitz W (2002) Curr Opin Chem Biol 6:243–249
Stadler C, De Lacey AL, Montet Y, Volbeda A, Fontecilla-Camps JC, Conesa JC, Fernandez VM (2002) Inorg Chem 41:4424–4434
Cammack R, Fernández VM, Hatchikian EC (1994) Methods Enzymol 243:43–68
Dementin S, Burlat B, DeLacey AL, Pardo A, Adryanczyk-Perrier G, Guigliarelli B, Fernández VM, Rousset M (2004) J Biol Chem 279:10508–10513
Covington AK, Paabo M, Robinson RA, Bates RG (1968) Anal Chem 40:700–706
Moss D, Nabedryk E, Breton J, Mäntele W (1990) Eur J Biochem 187:565–572
Van der Spek TM, Arendsen AF, Happe RP, Yun S, Kimberley KA, Stufkens DJ, Hagen WR, Albracht SPJ (1996) Eur J Biochem 237:629–634
De Lacey AL, Stadler C, Fernandez VM, Hatchikian EC, Fan HJ, Li S, Hall MB (2002) J Biol Inorg Chem 7:318–326
Hatchikian EC, Traore AS, Fernandez VM, Cammack R (1990) Eur J Biochem 187:635–643
Rousset M, Montet Y, Guigliarelli B, Forget N, Asso M, Bertrand P, Fontecilla-Camps JC, Hatchikian EC (1998) Proc Natl Acad Sci USA 95:11625–11630
De Lacey AL, Fernandez VM, Rousset M, Cavazza C, Hatchikian EC (2003) J Biol Inorg Chem 8:129–134
Hille R (1991) Biochemistry 30:8522–8529
Bishop GR, Davidson VL (1995) Biochemistry 34:12082–12086
Glickman MH, Klinman JP (1995) Biochemistry 34:14077–14092
Acknowledgements
This work was supported by the Spanish MCYT (project BQU2003-04221) and CAM (project 07N/0068/2002) and by grant G5RD-CT-2002-00750 from the European Commission Competitive and Sustainable Growth Programme. M.R. is “Laboratoire de Recherche Conventionné” with the CEA, no. 25V. We thank Dr A. Volbeda for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
De Lacey, A.L., Pardo, A., Fernández, V.M. et al. FTIR spectroelectrochemical study of the activation and inactivation processes of [NiFe] hydrogenases: effects of solvent isotope replacement and site-directed mutagenesis. J Biol Inorg Chem 9, 636–642 (2004). https://doi.org/10.1007/s00775-004-0559-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-004-0559-7