Abstract
Introduction
Irisin is a proteolytic product of fibronectin type II domain-containing 5, which is related to the improvement in glucose metabolism. Numerous studies have suggested that irisin is a crucial myokine linking muscle to bone in physiological and pathophysiological states.
Materials and methods
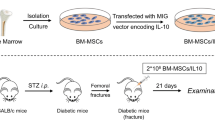
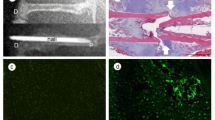
We examined the effects of local irisin administration with gelatin hydrogel sheets and intraperitoneal injection of irisin on the delayed femoral bone repair caused by streptozotocin (STZ)-induced diabetes in female mice. We analyzed the femurs of mice using quantitative computed tomography and histological analyses and then measured the mRNA levels in the damaged mouse tissues.
Results
Local irisin administration significantly blunted the delayed bone repair induced by STZ 10 days after a femoral bone defect was generated. Local irisin administration significantly blunted the number of Osterix-positive cells that were suppressed by STZ at the damaged site 4 days after a femoral bone defect was generated, although it did not affect the mRNA levels of chondrogenic and adipogenic genes 4 days after bone injury in the presence or absence of diabetes. On the other hand, intraperitoneal injection of irisin did not affect delayed bone repair induced by STZ 10 days after bone injury. Irisin significantly blunted the decrease in Osterix mRNA levels induced by advanced glycation end products or high-glucose conditions in ST2 cells in the presence of bone morphogenetic protein-2.
Conclusions
We first showed that local irisin administration with gelatin hydrogel sheets improves the delayed bone repair induced by diabetic state partially by enhancing osteoblastic differentiation.






Similar content being viewed by others
References
Kawao N, Kaji H (2015) Interactions between muscle tissues and bone metabolism. J Cell Biochem 116:687–695
Kaji H (2016) Effects of myokines on bone. Bonekey Rep 5:826
Nie Y, Dai B, Guo X, Liu D (2020) Cleavage of FNDC5 and insights into its maturation process. Mol Cell Endocrinol 510:110840
Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y et al (2018) Irisin mediates effects on bone and fat via alphaV integrin receptors. Cell 175:1756–1768
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM (2012) A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481:463–468
Zhou K, Qiao X, Cai Y, Li A, Shan D (2019) Lower circulating irisin in middle-aged and older adults with osteoporosis: a systematic review and meta-analysis. Menopause 26:1302–1310
Wu LF, Zhu DC, Tang CH, Ge B, Shi J et al (2018) Association of plasma irisin with bone mineral density in a large Chinese population using an extreme sampling design. Calcif Tissue Int 103:246–251
Anastasilakis AD, Polyzos SA, Makras P, Gkiomisi A, Bisbinas I, Katsarou A, Filippaios A, Mantzoros CS (2014) Circulating irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not affected by either teriparatide or denosumab treatment for 3 months. Osteoporos Int 25:1633–1642
Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C et al (2015) The myokine irisin increases cortical bone mass. Proc Natl Acad Sci USA 112:12157–12162
Colaianni G, Mongelli T, Cuscito C, Pignataro P, Lippo L, Spiro G, Notarnicola A, Severi I, Passeri G, Mori G, Brunetti G, Moretti B, Tarantino U, Colucci SC, Reseland JE, Vettor R, Cinti S, Grano M (2017) Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci Rep 7:2811
Kawao N, Moritake A, Tatsumi K, Kaji H (2018) Roles of irisin in the linkage from muscle to bone during mechanical unloading in mice. Calcif Tissue Int 103:24–34
Kawao N, Iemura S, Kawaguchi M, Mizukami Y, Takafuji Y, Kaji H (2021) Role of irisin in effects of chronic exercise on muscle and bone in ovariectomized mice. J Bone Miner Metab 39:547–557
Tamura Y, Kawao N, Shimoide T, Okada K, Matsuo O, Kaji H (2018) Role of plasminogen activator inhibitor-1 in glucocorticoid-induced muscle change in mice. J Bone Miner Metab 36:148–156
Tamura Y, Fujito H, Kawao N, Kaji H (2017) Vitamin D deficiency aggravates diabetes-induced muscle wasting in female mice. Diabetol Int 8:52–58
Iemura S, Kawao N, Okumoto K, Akagi M, Kaji H (2020) Role of irisin in androgen-deficient muscle wasting and osteopenia in mice. J Bone Miner Metab 38:161–171
Chen Z, Zhang Y, Zhao F, Yin C, Yang C, Wang X, Wu Z, Liang S, Li D, Lin X, Tian Y, Hu L, Li Y, Qian A (2020) Recombinant irisin prevents the reduction of osteoblast differentiation induced by stimulated microgravity through increasing beta-Catenin expression. Int J Mol Sci 21:1259
Colaianni G, Cuscito C, Mongelli T, Oranger A, Mori G, Brunetti G, Colucci S, Cinti S, Grano M (2014) Irisin enhances osteoblast differentiation in vitro. Int J Endocrinol 2014:902186
Qiao X, Nie Y, Ma Y, Chen Y, Cheng R, Yin W, Hu Y, Xu W, Xu L (2016) Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci Rep 6:18732
Estell EG, Le PT, Vegting Y, Kim H, Wrann C, Bouxsein ML, Nagano K, Baron R, Spiegelman BM, Rosen CJ (2020) Irisin directly stimulates osteoclastogenesis and bone resorption in vitro and in vivo. Elife 9:e58172
Storlino G, Colaianni G, Sanesi L, Lippo L, Brunetti G, Errede M, Colucci S, Passeri G, Grano M (2020) Irisin prevents disuse-induced osteocyte apoptosis. J Bone Miner Res 35:766–775
He Z, Li H, Han X, Zhou F, Du J, Yang Y, Xu Q, Zhang S, Zhang S, Zhao N, Yan M (2020) Irisin inhibits osteocyte apoptosis by activating the Erk signaling pathway in vitro and attenuates ALCT-induced osteoarthritis in mice. Bone 141:115573
Ma Y, Qiao X, Zeng R, Cheng R, Zhang J, Luo Y, Nie Y, Hu Y, Yang Z, Zhang J, Liu L, Xu W, Xu CC, Xu L (2018) Irisin promotes proliferation but inhibits differentiation in osteoclast precursor cells. FASEB J. https://doi.org/10.1096/fj.201700983RR
Claes L, Recknagel S, Ignatius A (2012) Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol 8:133–143
Hu K, Olsen BR (2016) Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest 126:509–526
Xin X, Wu J, Zheng A, Jiao D, Liu Y, Cao L, Jiang X (2019) Delivery vehicle of muscle-derived irisin based on silk/calcium silicate/sodium alginate composite scaffold for bone regeneration. Int J Nanomedicine 14:1451–1467
Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes -a meta-analysis. Osteoporos Int 18:427–444
Retzepi M, Donos N (2010) The effect of diabetes mellitus on osseous healing. Clin Oral Implants Res 21:673–681
Shimoide T, Kawao N, Tamura Y, Okada K, Horiuchi Y, Okumoto K, Kurashimo S, Ishida M, Tatsumi K, Matsuo O, Kaji H (2018) Role of macrophages and plasminogen activator inhibitor-1 in delayed bone repair in diabetic female mice. Endocrinology 159:1875–1885
Mao L, Kawao N, Tamura Y, Okumoto K, Okada K, Yano M, Matsuo O, Kaji H (2014) Plasminogen activator inhibitor-1 is involved in impaired bone repair associated with diabetes in female mice. PLoS ONE 9:e92686
Gamas L, Matafome P, Seica R (2015) Irisin and myonectin regulation in the insulin resistant muscle: implications to adipose tissue: muscle crosstalk. J Diabetes Res 2015:359159
Yang Z, Chen X, Chen Y, Zhao Q (2015) Decreased irisin secretion contributes to muscle insulin resistance in high-fat diet mice. Int J Clin Exp Pathol 8:6490–6497
Okada K, Okamoto T, Okumoto K, Takafuji Y, Ishida M, Kawao N, Matsuo O, Kaji H (2020) PAI-1 is involved in delayed bone repair induced by glucocorticoids in mice. Bone 134:115310
Mishima S, Takahashi K, Kiso H, Murashima-Suginami A, Tokita Y, Jo JI, Uozumi R, Nambu Y, Huang B, Harada H, Komori T, Sugai M, Tabata Y, Bessho K (2021) Local application of Usag-1 siRNA can promote tooth regeneration in Runx2-deficient mice. Sci Rep 11:13674
Hong L, Jing S, Tong W, Jiangying K, Qinhui L, Shihai C, Shiyun P, Lei C, Rui L, Yanping L, Min Z, Zhiyong Z, Wei J, Aijuan Q, Jinhan H (2019) Irisin is controlled by farnesoid X receptor and regulates cholesterol homeostasis. Front Pharmacol 10:548
Tanaka K, Yamaguchi T, Kaji H, Kanazawa I, Sugimoto T (2013) Advanced glycation end products suppress osteoblastic differentiation of stromal cells by activating endoplasmic reticulum stress. Biochem Biophys Res Commun 438:463–467
Swaroop JJ, Rajarajeswari D, Naidu JN (2012) Association of TNF-alpha with insulin resistance in type 2 diabetes mellitus. Indian J Med Res 135:127–130
Huebschmann AG, Regensteiner JG, Vlassara H, Reusch JE (2006) Diabetes and advanced glyco-oxidation end products. Diabetes Care 29:1420–1432
Zuo CJ, Zhao XY, Shi Y, Wu W, Zhang N, Xu J, Wang C, Hu G, Zhang X (2018) TNF-alpha inhibits SATB2 expression and osteoblast differentiation through NF-kappa B and MAPK pathways. Oncotarget 9:4833–4850
Liu CL, Jiang DM (2017) High glucose-induced LIF suppresses osteoblast differentiation via regulating STAT3/SOCS3 signaling. Cytokine 91:132–139
Gandhi A, Beam HA, O’Connor JP, Parsons JR, Lin SS (2005) The effects of local insulin delivery on diabetic fracture healing. Bone 37:482–490
Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM (2009) Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukar Gene 19:109–124
McCabe LR (2007) Understanding the pathology and mechanisms of type I diabetic bone loss. J Cell Biochem 102:1343–1357
Li X, Zhu X, Wu H, Van Dyke TE, Xu X, Morgan EF, Fu W, Liu C, Tu Q, Huang D, Chen J (2021) Roles and mechanisms of irisin in attenuating pathological features of osteoarthritis. Front Cell Dev Biol 9:703670
Kawao N, Kawaguchi M, Ohira T, Ehara H, Mizukami Y, Takafuji Y, Kaji H (2022) Renal failure suppresses muscle irisin expression and irisin blunts cortical bone loss in mice. J Cachexia Sarcopenia Muscle 13:758–771
Acknowledgements
The study was partially supported by grants from The Osaka Medical Research Foundation for Intractable Diseases to Y.T., the Cooperative Research Program (Joint usage/Research Center program) of the Institute for Frontier Life and Medical Sciences, Kyoto University, to Y.T., a JSPS KAKENHI Grant-in-Aid for Early Career Scientists (19K18480) and a Grant-in-Aid for Scientific Research (C: 21K09240) to Y.T., as well as a Grant-in-Aid for Scientific Research on Innovative Areas (15H05935, “Living in Space”)/Grant-in-Aid for Scientific Research (C:20K09514) to H.K. from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Contributions
YK: investigation, formal analysis, and writing—original draft. YT: conceptualization, investigation, supervision, writing—review and editing, and funding acquisition. KO: resources and investigation. YT: investigation. HE: resources and investigation. YM: resources. NK: conceptualization. JJ: resources. YT: resources and writing—review and editing. HK: conceptualization, supervision, writing—review and editing, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kinoshita, Y., Takafuji, Y., Okumoto, K. et al. Irisin improves delayed bone repair in diabetic female mice. J Bone Miner Metab 40, 735–747 (2022). https://doi.org/10.1007/s00774-022-01353-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-022-01353-3