Abstract
Introduction
Osteoarthritis is a common joint disease that causes destruction of articular cartilage and severe inflammation surrounding knee and hip joints. However, to date, effective therapeutic reagents for osteoarthritis have not been developed because the underlying molecular mechanisms are complex. Recent genetic findings suggest that a Wnt antagonist, frizzled-related protein B (FRZB), is a potential therapeutic target for osteoarthritis. Therefore, this study aimed to examine the transcriptional regulation of FRZB in chondrocytes.
Materials and methods
Frzb/FRZB expression was assessed by RT-qPCR analyses in murine articular chondrocytes and SW1353 chondrocyte cell line. Overexpression and knockdown experiments were performed using adenovirus and lentivirus, respectively. Luciferase-reporter and chromatin immunoprecipitation assays were performed for determining transcriptional regulation. Protein–protein interaction was determined by co-immunoprecipitation analysis.
Results
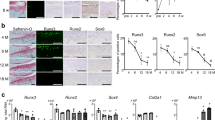
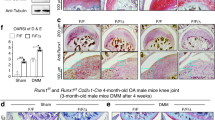
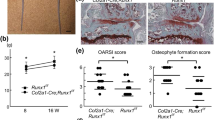
Frzb was highly expressed in cartilages, especially within articular chondrocytes. Interleukin-1α markedly reduced Frzb expression in articular chondrocytes in association with cartilage destruction and increases in ADAM metallopeptidase with thrombospondin type 1 motif (Adamts) 4 and Adamts5 expression. Bone morphogenetic protein 2 (BMP2) increased FRZB expression in SW1353 cells through Smad signaling. Osterix and msh homeobox 2 (Msx2), both of which function as downstream transcription factors of BMP2, induced FRZB expression and upregulated its promoter activity. Co-immunoprecipitation results showed a physical interaction between Osterix and Msx2. Knockdown of either Osterix or Msx2 inhibited BMP2-dependent FRZB expression. Chromatin immunoprecipitation indicated a direct association of Osterix and Msx2 with the FRZB gene promoter.
Conclusion
These results suggest that BMP2 regulates FRZB expression through Osterix and Msx2.





Similar content being viewed by others
References
Yoshimura N, Muraki S, Oka H, Mabuchi A, En-Yo Y, Yoshida M, Saika A, Yoshida H, Suzuki T, Yamamoto S, Ishibashi H, Kawaguchi H, Nakamura K, Akune T (2009) Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study (in eng). J Bone Miner Metab 27:620–628. https://doi.org/10.1007/s00774-009-0080-8
Creamer P, Hochberg MC (1997) Osteoarthritis (in eng). Lancet (London, England) 350:503–508. https://doi.org/10.1016/s0140-6736(97)07226-7
Schumacher BL, Block JA, Schmid TM, Aydelotte MB, Kuettner KE (1994) A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage (in eng). Arch Biochem Biophys 311:144–152. https://doi.org/10.1006/abbi.1994.1219
Saito T, Nishida K, Furumatsu T, Yoshida A, Ozawa M, Ozaki T (2013) Histone deacetylase inhibitors suppress mechanical stress-induced expression of RUNX-2 and ADAMTS-5 through the inhibition of the MAPK signaling pathway in cultured human chondrocytes (in eng). Osteoarthritis Cartilage 21:165–174. https://doi.org/10.1016/j.joca.2012.09.003
Pincus T, Wang X, Chung C, Sokka T, Koch GG (2005) Patient preference in a crossover clinical trial of patients with osteoarthritis of the knee or hip: face validity of self-report questionnaire ratings (in eng). J Rheumatol 32:533–539
Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, Carr AJ (2015) Osteoarthritis (in eng). Lancet (London, England) 386:376–387. https://doi.org/10.1016/s0140-6736(14)60802-3
Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM (1997) Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer (in eng). Cell 88:747–756. https://doi.org/10.1016/s0092-8674(00)81921-2
Kawaguchi H (2009) Regulation of osteoarthritis development by Wnt-beta-catenin signaling through the endochondral ossification process (in eng). J Bone Miner Res: Off J Am Soc Bone Miner Res 24:8–11. https://doi.org/10.1359/jbmr.081115
Loughlin J, Dowling B, Chapman K, Marcelline L, Mustafa Z, Southam L, Ferreira A, Ciesielski C, Carson DA, Corr M (2004) Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females (in eng). Proc Natl Acad Sci USA 101:9757–9762. https://doi.org/10.1073/pnas.0403456101
Lories RJ, Peeters J, Bakker A, Tylzanowski P, Derese I, Schrooten J, Thomas JT, Luyten FP (2007) Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice (in eng). Arthritis Rheum 56:4095–4103. https://doi.org/10.1002/art.23137
Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, Rosier RN, O’Keefe RJ, Zuscik M, Chen D (2009) Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice (in eng). J Bone Miner Res: Off J Am Soc Bone Miner Res 24:12–21. https://doi.org/10.1359/jbmr.080901
Ono K, Hata K, Nakamura E, Ishihara S, Kobayashi S, Nakanishi M, Yoshida M, Takahata Y, Murakami T, Takenoshita S, Komori T, Nishimura R, Yoneda T (2021) Dmrt2 promotes transition of endochondral bone formation by linking Sox9 and Runx2 (in eng). Commun Biol 4:326. https://doi.org/10.1038/s42003-021-01848-1
Amano K, Hata K, Muramatsu S, Wakabayashi M, Takigawa Y, Ono K, Nakanishi M, Takashima R, Kogo M, Matsuda A, Nishimura R, Yoneda T (2011) Arid5a cooperates with Sox9 to stimulate chondrocyte-specific transcription (in eng). Mol Biol Cell 22:1300–1311. https://doi.org/10.1091/mbc.E10-07-0566
Takigawa Y, Hata K, Muramatsu S, Amano K, Ono K, Wakabayashi M, Matsuda A, Takada K, Nishimura R, Yoneda T (2010) The transcription factor Znf219 regulates chondrocyte differentiation by assembling a transcription factory with Sox9 (in eng). J Cell Sci 123:3780–3788. https://doi.org/10.1242/jcs.071373
Nishimura R, Hata K, Matsubara T, Wakabayashi M, Yoneda T (2012) Regulation of bone and cartilage development by network between BMP signalling and transcription factors (in eng). J Biochem 151:247–254. https://doi.org/10.1093/jb/mvs004
Stanton H, Golub SB, Rogerson FM, Last K, Little CB, Fosang AJ (2011) Investigating ADAMTS-mediated aggrecanolysis in mouse cartilage (in eng). Nat Protoc 6:388–404. https://doi.org/10.1038/nprot.2010.179
Takahata Y, Nakamura E, Hata K, Wakabayashi M, Murakami T, Wakamori K, Yoshikawa H, Matsuda A, Fukui N, Nishimura R (2019) Sox4 is involved in osteoarthritic cartilage deterioration through induction of ADAMTS4 and ADAMTS5 (in eng). FASEB J: Off Publ Fed Am Soc Exp Biol 33:619–630. https://doi.org/10.1096/fj.201800259R
Gebauer M, Saas J, Sohler F, Haag J, Söder S, Pieper M, Bartnik E, Beninga J, Zimmer R, Aigner T (2005) Comparison of the chondrosarcoma cell line SW1353 with primary human adult articular chondrocytes with regard to their gene expression profile and reactivity to IL-1beta (in eng). Osteoarthritis Cartilage 13:697–708. https://doi.org/10.1016/j.joca.2005.04.004
Nishimura R, Hata K, Ikeda F, Ichida F, Shimoyama A, Matsubara T, Wada M, Amano K, Yoneda T (2008) Signal transduction and transcriptional regulation during mesenchymal cell differentiation (in eng). J Bone Miner Metab 26:203–212. https://doi.org/10.1007/s00774-007-0824-2
Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K (1997) Smad6 inhibits signalling by the TGF-beta superfamily (in eng). Nature 389:622–626. https://doi.org/10.1038/39355
Matsubara T, Kida K, Yamaguchi A, Hata K, Ichida F, Meguro H, Aburatani H, Nishimura R, Yoneda T (2008) BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation (in eng). J Biol Chem 283:29119–29125. https://doi.org/10.1074/jbc.M801774200
Akiyama H, Lefebvre V (2011) Unraveling the transcriptional regulatory machinery in chondrogenesis (in eng). J Bone Miner Metab 29:390–395. https://doi.org/10.1007/s00774-011-0273-9
Gamer LW, Pregizer S, Gamer J, Feigenson M, Ionescu A, Li Q, Han L, Rosen V (2018) The Role of Bmp2 in the Maturation and Maintenance of the Murine Knee Joint (in eng). J Bone Miner Res: Off J Am Soc Bone Miner Res 33:1708–1717. https://doi.org/10.1002/jbmr.3441
Yoshida CA, Yamamoto H, Fujita T, Furuichi T, Ito K, Inoue K, Yamana K, Zanma A, Takada K, Ito Y, Komori T (2004) Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog (in eng). Genes Dev 18:952–963. https://doi.org/10.1101/gad.1174704
Komori T (2020) Molecular Processes in Chondrocyte Biology (in eng). Int J Mol Sci. https://doi.org/10.3390/ijms21114161
van Beuningen HM, Glansbeek HL, van der Kraan PM, van den Berg WB (1998) Differential effects of local application of BMP-2 or TGF-beta 1 on both articular cartilage composition and osteophyte formation (in eng). Osteoarthritis Cartilage 6:306–317. https://doi.org/10.1053/joca.1998.0129
Zhou Y, Wang T, Hamilton JL, Chen D (2017) Wnt/β-catenin signaling in osteoarthritis and in other forms of arthritis (in eng). Curr Rheumatol Rep 19:53. https://doi.org/10.1007/s11926-017-0679-z
Plotz B, Bomfim F, Sohail MA, Samuels J (2021) Current epidemiology and risk factors for the development of hand osteoarthritis (in eng). Curr Rheumatol Rep 23:61. https://doi.org/10.1007/s11926-021-01025-7
Wehmeyer C, Frank S, Beckmann D, Böttcher M, Cromme C, König U, Fennen M, Held A, Paruzel P, Hartmann C, Stratis A, Korb-Pap A, Kamradt T, Kramer I, van den Berg W, Kneissel M, Pap T, Dankbar B (2016) Sclerostin inhibition promotes TNF-dependent inflammatory joint destruction (in eng). Sci Transl Med 8:330ra35. https://doi.org/10.1126/scitranslmed.aac4351
Nakamura Y, Nawata M, Wakitani S (2005) Expression profiles and functional analyses of Wnt-related genes in human joint disorders (in eng). Am J Pathol 167:97–105. https://doi.org/10.1016/s0002-9440(10)62957-4
Dell’accio F, De Bari C, Eltawil NM, Vanhummelen P, Pitzalis C (2008) Identification of the molecular response of articular cartilage to injury, by microarray screening: Wnt-16 expression and signaling after injury and in osteoarthritis (in eng). Arthritis Rheum 58:1410–1421. https://doi.org/10.1002/art.23444
Papathanasiou I, Malizos KN, Tsezou A (2012) Bone morphogenetic protein-2-induced Wnt/β-catenin signaling pathway activation through enhanced low-density-lipoprotein receptor-related protein 5 catabolic activity contributes to hypertrophy in osteoarthritic chondrocytes (in eng). Arthritis Res Ther 14:R82. https://doi.org/10.1186/ar3805
Iwakura T, Inui A, Reddi AH (2013) Stimulation of superficial zone protein accumulation by hedgehog and Wnt signaling in surface zone bovine articular chondrocytes (in eng). Arthritis Rheum 65:408–417. https://doi.org/10.1002/art.37768
Kitagaki J, Iwamoto M, Liu JG, Tamamura Y, Pacifci M, Enomoto-Iwamoto M (2003) Activation of beta-catenin-LEF/TCF signal pathway in chondrocytes stimulates ectopic endochondral ossification (in eng). Osteoarthritis Cartilage 11:36–43. https://doi.org/10.1053/joca.2002.0863
Saito T, Fukai A, Mabuchi A, Ikeda T, Yano F, Ohba S, Nishida N, Akune T, Yoshimura N, Nakagawa T, Nakamura K, Tokunaga K, Chung UI, Kawaguchi H (2010) Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development (in eng). Nat Med 16:678–686. https://doi.org/10.1038/nm.2146
Nishimura R, Hata K, Nakamura E, Murakami T, Takahata Y (2018) Transcriptional network systems in cartilage development and disease (in eng). Histochem Cell Biol 149:353–363. https://doi.org/10.1007/s00418-017-1628-7
Yasuhara R, Ohta Y, Yuasa T, Kondo N, Hoang T, Addya S, Fortina P, Pacifici M, Iwamoto M, Enomoto-Iwamoto M (2011) Roles of β-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells (in eng). Lab Invest J Tech Methods Pathol 91:1739–52. https://doi.org/10.1038/labinvest.2011.144
Acknowledgements
We thank Addgene for providing pCMV-VSV-G, pRSV-Rev, pMDLg/pRRE and XE143 sFRP-3-CS2+ plasmids.
Funding
This work was supported by Japan Society for the Promotion of Science grants-in-aid for scientific research (16H06393, 20K20475 and 21H04841).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Yagi, H., Takahata, Y., Murakami, T. et al. Transcriptional regulation of FRZB in chondrocytes by Osterix and Msx2. J Bone Miner Metab 40, 723–734 (2022). https://doi.org/10.1007/s00774-022-01345-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-022-01345-3