Abstract
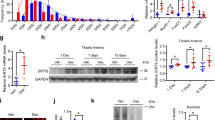
We recently revealed that plasminogen activator inhibitor-1 (PAI-1), a serine protease inhibitor, is involved in diabetes, osteoporosis and muscle wasting induced by glucocorticoid (GC) treatment in mice. In the present study, we investigated the detailed mechanisms by which GC induces muscle wasting through PAI-1 in vivo and in vitro. PAI-1 deficiency suppressed the mRNA levels of atrogin1 and muscle RING-Finger Protein 1 (MuRF1), ubiquitin ligases leading to muscle degradation, elevated by GC treatment in the gastrocnemius muscle of mice. In vitro study revealed that active PAI-1 treatment augmented the increase in atrogin1 mRNA levels enhanced by dexamethasone (Dex) in mouse myoblastic C2C12 cells. Moreover, a reduction in endogenous PAI-1 level by siRNA suppressed the mRNA levels of atrogin1 and MuRF1 enhanced by Dex in C2C12 cells. In contrast, a reduction in endogenous PAI-1 levels and active PAI-1 did not affect the phosphorylations of Akt and p70S6 kinase nor myogenic differentiation with or without Dex in C2C12 cells. In addition, PAI-1 deficiency blunted IGF-1 mRNA levels decreased by GC treatment in the gastrocnemius muscle of mice, although neither active PAI-1 nor a reduction in endogenous PAI-1 levels affected the levels of IGF-1 mRNA in C2C12 cells in the presence of Dex. In conclusion, our data suggest that paracrine PAI-1 is involved in GC-induced muscle wasting through the enhancement of muscle degradation in mice.






Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Rhen T, Cidlowski JA (2005) Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med 353:1711–1723
Strehl C, Buttgereit F (2013) Optimized glucocorticoid therapy: teaching old drugs new tricks. Mol Cell Endocrinol 380:32–40
van Raalte DH, Ouwens DM, Diamant M (2009) Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest 39:81–93
Kuo T, Harris CA, Wang JC (2013) Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol Cell Endocrinol 380:79–88
Henneicke H, Gasparini SJ, Brennan-Speranza TC, Zhou H, Seibel MJ (2014) Glucocorticoids and bone: local effects and systemic implications. Trends Endocrinol Metab 25:197–211
Hasselgren PO, Alamdari N, Aversa Z, Gonnella P, Smith IJ, Tizio S (2010) Corticosteroids and muscle wasting: role of transcription factors, nuclear cofactors, and hyperacetylation. Curr Opin Clin Nutr Metab Care 13:423–428
Braun TP, Marks DL (2015) The regulation of muscle mass by endogenous glucocorticoids. Front Physiol 6:12
Kawao N, Kaji H (2015) Interactions between muscle tissues and bone metabolism. J Cell Biochem 116:687–695
Kaji H (2016) Effects of myokines on bone. BoneKEy Rep 5:826
Waddell DS, Baehr LM, van den Brandt J, Johnsen SA, Reichardt HM, Furlow JD, Bodine SC (2008) The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol Endocrinol Metab 295:E785–E797
Castillero E, Alamdari N, Lecker SH, Hasselgren PO (2013) Suppression of Atrogin1 and MuRF1 prevents dexamethasone-induced atrophy of cultured myotubes. Metabolism 62:1495–1502
Goodman CA, Mayhew DL, Hornberger TA (2011) Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal 23:1896–1906
Wang H, Kubica N, Ellisen LW, Jefferson LS, Kimball SR (2006) Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J Biol Chem 281:39128–39134
Wu Y, Zhao W, Zhao J, Zhang Y, Qin W, Pan J, Bauman WA, Blitzer RD, Cardozo C (2010) REDD1 is a major target of testosterone action in preventing dexamethasone-induced muscle loss. Endocrinology 151:1050–1059
Dong Y, Pan JS, Zhang L (2013) Myostatin suppression of Akirin1 mediates glucocorticoid-induced satellite cell dysfunction. PLoS One 8:e58554
Hoekstra T, Geleijnse JM, Schouten EG, Kluft C (2004) Plasminogen activator inhibitor-type 1: its plasma determinants and relation with cardiovascular risk. Thromb Haemost 91:861–872
Ma LJ, Mao SL, Taylor KL, Kanjanabuch T, Guan Y, Zhang Y, Brown NJ, Swift LL, McGuinness OP, Wasserman DH, Vaughan DE, Fogo AB (2004) Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes 53:336–346
Mathieu P, Lemieux I, Despres JP (2010) Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther 87:407–416
Tamura Y, Kawao N, Okada K, Yano M, Okumoto K, Matsuo O, Kaji H (2013) Plasminogen activator inhibitor-1 is involved in streptozotocin-induced bone loss in female mice. Diabetes 62:3170–3179
Kaji H (2016) Adipose tissue-derived plasminogen activator inhibitor-1 function and regulation. Compr Physiol 6:1873–1896
Tamura Y, Kawao N, Yano M, Okada K, Okumoto K, Chiba Y, Matsuo O, Kaji H (2015) Role of plasminogen activator inhibitor-1 in glucocorticoid-induced diabetes and osteopenia in mice. Diabetes 64:2194–2206
Carmeliet P, Moons L, Lijnen R, Janssens S, Lupu F, Collen D, Gerard RD (1997) Inhibitory role of plasminogen activator inhibitor-1 in arterial wound healing and neointima formation: a gene targeting and gene transfer study in mice. Circulation 96:3180–3191
Tanaka K, Kanazawa I, Yamaguchi T, Yano S, Kaji H, Sugimoto T (2014) Active vitamin D possessed beneficial effects on the interaction between muscle and bone. Biochem Biophys Res Commun 450:482–487
Kawao N, Tamura Y, Okumoto K, Yano M, Okada K, Matsuo O, Kaji H (2014) Tissue-type plasminogen activator deficiency delays bone repair: roles of osteoblastic proliferation and vascular endothelial growth factor. Am J Physiol Endocrinol Metab 307:E278–E288
Lecker SH, Solomon V, Mitch WE, Goldberg AL (1999) Muscle protein breakdown and the critical role of the ubiquitin–proteasome pathway in normal and disease states. J Nutr 129:227S–237S
Kandarian SC, Jackman RW (2006) Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 33:155–165
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708
Bodine SC, Baehr LM (2014) Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/Atrogin1. Am J Physiol Endocrinol Metab 307:E469–E484
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117:399–41229
Baehr LM, Furlow JD, Bodine SC (2011) Muscle sparing in muscle RING finger 1 null mice: response to synthetic glucocorticoids. J Physiol 589:4759–4776
Barlovatz-Meimon G, Frisdal E, Hantai D, Angles-Cano E, Gautron J (1990) Slow and fast rat skeletal muscles differ in their plasminogen activator activities. Eur J Cell Biol 52:157–162
Quax PH, Frisdal E, Pedersen N, Bonavaud S, Thibert P, Martelly I, Verheijen JH, Blasi F, Barlovatz-Meimon G (1992) Modulation of activities and RNA level of the components of the plasminogen activation system during fusion of human myogenic satellite cells in vitro. Dev Biol 151:166–175
Suelves M, Lopez-Alemany R, Lluis F, Aniorte G, Serrano E, Parra M, Carmeliet P, Munoz-Canoves P (2002) Plasmin activity is required for myogenesis in vitro and skeletal muscle regeneration in vivo. Blood 99:2835–2844
Acknowledgements
The study was supported by grants-in-aid for scientific research 16K10924 and 15K08220 from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to Y.T. and H.K., respectively) and grants from the Takeda Science Foundation and the Osaka Medical Research Foundation for Intractable Diseases.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest.
About this article
Cite this article
Tamura, Y., Kawao, N., Shimoide, T. et al. Role of plasminogen activator inhibitor-1 in glucocorticoid-induced muscle change in mice. J Bone Miner Metab 36, 148–156 (2018). https://doi.org/10.1007/s00774-017-0825-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-017-0825-8